Abstract
Background:
Studies with short-term follow up have demonstrated favorable effects of weight loss (WL) on the heart, but little information is available regarding long-term effects, or effects of visceral fat reduction.
Objectives:
Evaluate the effects of long-term WL following bariatric surgery on cardiac structure, function, ventricular interaction, and body composition, including epicardial adipose thickness (EAT) and abdominal visceral adipose tissue (VAT).
Methods:
213 obese patients underwent echocardiography before and >180 days following bariatric surgery. Of these patients, abdominal VAT-area was measured by computed tomography in n=52.
Results:
After 5.3 (IQR 2.9–7.9) years, BMI decreased by 22%, with favorable reductions in blood pressure, fasting glucose, and LV remodeling in the full sample. In the subgroup of patients with abdominal CT VAT-area decreased by 30%. In all subjects, EAT was reduced by 14% (both p<0.0001) in tandem with reductions in ventricular interdependence. LV and RV longitudinal strain improved following WL, but left atrial (LA) strain deteriorated, while LA volume and estimated LA pressures increased. In subgroup analysis, LV wall thickness and strain correlated more strongly with VAT than BMI at baseline, and reductions in LV mass following surgery were correlated with decreases in VAT, but not BMI.
Conclusions:
In this observational study, weight loss following bariatric surgery was associated with epicardial fat reduction, reduced ventricular interaction, LV reverse remodeling, and improved longitudinal biventricular mechanics, but LA myopathy and hemodynamic congestion still progressed. Reduction in visceral fat was associated with favorable cardiac effects, suggesting this might be a key target of WL interventions.
Keywords: Obesity, Cardiac Function, Cardiac Structure, Bariatric surgery, heart failure
CONDENSED ABSTRACT:
We evaluated the effects of long-term weight loss following bariatric surgery. After median 5.3 years follow-up, BMI decreased by 22%, while VAT-area and EAT decreased by 30% and 14%. LV reverse remodeling and improvement of biventricular longitudinal strain were observed with reductions in ventricular interdependence. However, LA strain decreased and LA volume, and E/e’ ratio increased. Reductions in LV mass following surgery were correlated with decreases in VAT, but not BMI. These data reveal favorable chronic ventricular effects tied to visceral fat reduction, but also show that LA dysfunction may still progress despite robust weight loss.
INTRODUCTION
The prevalences of both obesity and heart failure (HF) with preserved ejection fraction (HFpEF) are increasing to epidemic proportions.1, 2 Obesity, especially when linked to increases in visceral fat, has deleterious effects on the cardiovascular system and is strongly tied to development and severity of HFpEF.3, 4 Excess body weight (BW) in patients with the obese phenotype of HFpEF is associated with greater cardiac hypertrophy, abnormal myocardial energetics, heightened pericardial restraint, and hemodynamic congestion that is more strongly linked to volume expansion and alterations in venous capacitance.4–7 These findings suggest that weight loss (WL) may improve these pathophysiologic components.
Modest WL via caloric restriction reduced left ventricular (LV) mass and improved exercise capacity in patients with obesity and HFpEF.8 Effects on cardiac function were minimal, but the treatment duration was short, and the degree of WL achieved modest. Bariatric surgery produces more robust and sustained WL, is associated with decreased risk of new-onset HF,9 and has been associated with LV and left atrial (LA) reverse remodeling in short term studies.10–13 However, very little is known regarding chronic effects of WL on the heart (>3 years), and existing literature is limited by smaller, highly-selected studies, variable selection criteria and imaging techniques, and inconsistent breadth of cardiac and body composition assessments.
The present study aimed to fill these gaps by evaluating the relationships between general adiposity, regional fat distribution, and cardiac structure, function, and ventricular interaction among obese adults without HF before and following bariatric surgery, with much longer follow up duration than previously reported.
METHODS
Study Population
This is a retrospective, observational cohort study evaluating consecutive adults who underwent bariatric surgery (including open and endoscopic surgeries) at the Mayo Clinic between January 2008 and December 2017. Patients who underwent an echocardiographic evaluation before and >180 days following bariatric surgery were included in the analysis to evaluate longitudinal changes. If a patient had multiple post-surgery echocardiograms, the most recent study (distant from Exam 1) was used as Exam 2. Exclusion criteria included patients who underwent repair surgery, with any history of LV ejection fraction (LVEF) <50%, primary cardiomyopathy, or moderate or greater valve disease. Of these patients, 52 underwent abdominal computed tomography before and after bariatric surgery within 12 months of echocardiography. Informed written consent was obtained from all patients to utilize their data, and the study was approved by the Mayo Clinic Institutional Review. The authors had full access to the data and take responsibility for its integrity.
Echocardiographic Assessment of Cardiac Structure and Function
Two-dimensional (2D), M-mode, Doppler and tissue Doppler echocardiography was performed pre-bariatric surgery and post-bariatric surgery according to the guidelines of the American Society of Echocardiography.14 LV mass was indexed to height.2.7 Relative wall thickness (RWT) was defined as the ratio of twice LV diastolic posterior wall thickness to the left ventricular end-diastolic dimension. LV end-diastolic volume, end-systolic volume, and LVEF were determined using the Teichholz method. Endocardial fractional shortening (eFS) was determined from 2- dimensional systolic and diastolic dimensions. LV diastolic function was assessed using the early diastolic mitral inflow velocity (E), the early diastolic septal mitral annular tissue velocity (e’), and the ratio of E/e’. Left atrial (LA) volume was determined using the biplane method of disks.
Right ventricular (RV) basal, mid-cavity, and longitudinal dimensions were measured at end-diastole using RV-focused views. RV fractional area change (FAC) was measured as an endocardial-based metric of RV systolic function.14 Myocardial deformation analyses were performed offline, using commercially available software (Image Arena, Tom Tec Imaging Systems, Unterschleissheim, Germany). LV global longitudinal strain (GLS) was measured using 2D speckle tracking. LVGLS was determined as the average of the three apical views. LA reservoir function was evaluated by the peak LA strain during LA relaxation using the QRS complex of electrocardiogram as fiducial point. RVGLS and RV free wall longitudinal strain (RVFWLS) were obtained from the apical 4 chamber view.15 Strain data are presented as absolute values since the direction of tissue motion during systole is self-evident. Echocardiographic and abdominal CT measurements were performed prospectively on existing images that were obtained during the predefined study interval by an experienced investigator who was blinded to patient information and the time point of the examination.
Assessment of Adipose Depots and Ventricular Interaction
Epicardial adipose tissue thickness (EAT) was measured perpendicularly to the free wall of the RV by echocardiography in the parasternal long-axis view.16 Total heart volume was estimated from two hemi-ellipsoids containing both atria and ventricles with the apical four-chamber view.17 Ventricular interdependence was quantified in the parasternal short-axis view on 2D echocardiography by the LV eccentricity index and using planimetry to calculate idealized and actual LV radii (ideal/actual radius), whereby higher values of both indices indicated greater septal flattening, enhanced ventricular interdependence, and increased pericardial restraint.6, 16
Among participants that underwent abdominal computed tomography scans for clinical indications within 12 months of echocardiography, abdominal visceral adipose tissue (VAT) area was measured at the L3 level based on the tomographic cross-sectional areas, as previously described (Supplemental Methods S1, Supplemental Figure 1).4, 18 The reader was blinded to the patient information and the time point of the examination.
The reliability and reproducibility of echocardiographic variable measurement was assessed in 20 randomly selected patients. Intra-observer agreement was evaluated after the same observer repeated the measurements 4 weeks later, and inter-observer agreement was tested by comparing the measurement made by another experienced reader. The intraclass correlation coefficients for intra-observer and inter-observer agreement were strong (all 0.72–0.95, Supplemental Table 1).
Statistical Analysis
Data are presented as mean (standard deviation, SD), median (interquartile range), or number (%). Within-group differences were assessed using paired t-test, Wilcoxon matched-pairs signed-ranks test or McNemar test as appropriate. Between-group differences were compared using the unpaired t-test, Wilcoxon rank-sum test, χ2 or Fisher’s exact test as appropriate. Pearson’s or Speaman’s correlation analysis was used to assess the relationships between continuous variables. Differences of correlation coefficients were assessed using a Meng’s Z-test. A two-sided p-value of <0.05 was considered statistically significant. All data were analyzed using JMP14.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Subject Characteristics
A total of 213 patients met inclusion criteria (Figure 1). The average age was 54±11 years, average body mass index (BMI) was 45±10 kg/m2, and 67% of the patients were women. Patients displayed typical comorbidities associated with obesity, including high prevalence of hypertension, diabetes mellitus, dyslipidemia, and obstructive sleep apnea (Table 1). A minority (5%) of patients had history of clinically diagnosed HFpEF. Of 213 patients, 52 patients underwent serial abdominal CT scan. Patients referred for abdominal CT displayed lower hemoglobin, but other baseline characteristics were similar to patients not referred for CT (Supplemental Table 2). VAT-area and subcutaneous adipose tissue (SAT)-area were measured in this subset of 52 subjects; the average VAT-area was 301±129 cm2, and the average SAT-area was 505±191 cm2 (Table 2).
Figure 1: Patient Identification.
Flow diagram outlining patient exclusions to qualify for inclusion to arrive at the final sample of 213 patients with repeat assessments prior to and following bariatric surgery. TTE, transthoracic echocardiography; TEE, transesophageal echocardiography; LVEF, left ventricular ejection fraction; F/U, follow-up; HOCM, hypertrophic obstructive cardiomyopathy; AS, aortic valve stenosis.
Table 1.
Baseline characteristics
| Mean or N (%) | |
|---|---|
| Age (years) | 54±11 |
| Female (%) | 143 (67) |
| Height (cm) | 167±10 |
| Body weight (kg) | 128±30 |
| Body mass index (kg/m2) | 43 (39, 50) |
| Surgical form, n (%) | |
| Gastric bypass and Roux-en Y gastrectomy | 121 (57) |
| Longitudinal gastrectomy | 48 (22) |
| Placement of gastric band | 12 (6) |
| Biliopancreatic division with duodenal switch | 27 (13) |
| Gastric bypass and small intestine reconstruction | 5 (2) |
| Comorbidities, n (%) | |
| Hypertension | 161 (76) |
| Coronary artery disease | 39 (18) |
| Atrial fibrillation | 35 (16) |
| Diabetes mellitus | 87 (41) |
| Dyslipidemia | 126 (59) |
| Obstructive sleep apnea | 151 (71) |
| HFpEF | 11 (5) |
| Medications, n (%) | |
| ACEI or ARB | 98 (46) |
| Beta-blocker | 89 (42) |
| Ca-blocker | 40 (19) |
| Diuretics | 101 (47) |
| Laboratories | |
| Hemoglobin (mg/dl) | 13.6±2.6 |
| eGFR (ml/min/1.73m2) | 55±22 |
| Fasting Glucose (mg/dl) | 110 (98, 132) |
| HbA1c (%) | 6.0 (5.5, 7.2) |
Values are mean ± SD, median (interquartile range), or n (%). ACEIs or ARBs, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HFpEF, heart failure with preserved ejection fraction.
Table 2.
Longitudinal Changes in Body Composition and Cardiometabolic Risk Factors
| Exam 1 | Exam 2 | P-value Exam 1 vs Exam 2 |
Change from baseline | |
|---|---|---|---|---|
| Follow-up duration (years) | 5.3 (2.9, 7.9) | |||
| Body Compositions | ||||
| Body height (cm) | 168±10 | 167±10 | <0.0001 | −1.0±3.3 |
| Body weight (kg) | 123 (106, 141) | 93 (80, 110) | <0.0001 | −31±25 |
| Body mass index (kg/m2) | 43 (39, 50) | 33 (29, 39) | <0.0001 | −11±9 |
| VAT-area (cm2), n=52 | 296 (216, 394) | 175 (94, 302) | <0.0001 | −92±117 |
| SAT-area (cm2), n=52 | 483 (367, 617) | 309 (224, 400) | <0.0001 | −176±181 |
| Estimated plasma volume (ml) , n=195 | 4018 (3497, 4664) | 3289 (2899, 3728) | <0.0001 | −785±744 |
| Estimated plasma volume/BSA (mL/m2), n=195 | 1757 (1644, 1498) | 1638 (1519, 1764) | <0.0001 | −157±221 |
| Comorbidities, n (%) | ||||
| Hypertension | 161 (76) | 156 (73) | 0.4 | −5 (−3) |
| Diabetes | 87 (41) | 49 (23) | <0.0001 | −38 (−18) |
| Obstructive sleep apnea | 151 (71) | 115 (54) | <0.0001 | −36 (−17) |
| Atrial fibrillation | 35 (16) | 48 (23) | 0.0003 | +13 (+7) |
| HFpEF | 11 (5) | 20 (9) | 0.003 | +9 (+4) |
| Medications, n (%) | ||||
| ACEI or ARB | 98 (46) | 92 (43) | 0.3 | −6 (−3) |
| Beta-blocker | 89 (42) | 85 (40) | 0.5 | −4 (−2) |
| Ca-blocker | 40 (19) | 39 (18) | 0.8 | −1 (−1) |
| Diuretics | 101 (47) | 83 (39) | 0.003 | −18 (−8) |
| Laboratories | ||||
| eGFR (ml/min/1.73m2), n=195 | 55±22 | 56±24 | 0.3 | 1.4±18.2 |
| Fasting Glucose (mg/dl), n=187 | 110 (98, 132) | 106 (90, 114) | <0.0001 | −15±36 |
| HbA1c (%), n=159 | 6.0 (5.5, 7.2) | 5.6 (5.1, 6.6) | <0.0001 | −0.6±1.3 |
| Hemodynamics | ||||
| Systolic BP (mmHg) | 131±19 | 125±20 | 0.0006 | −6±23 |
| Diastolic BP (mmHg) | 75±11 | 72±12 | 0.0003 | −4±14 |
| Heart rate (bpm) | 72 (64, 82) | 68 (60, 78) | 0.002 | −4±18 |
BP, blood pressure; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BSA, body surface area, HFpEF, heart failure with preserved ejection fraction; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; BP, blood pressure.
Sample size with available data shown in column 1 for assessments not available in all participants.
Baseline Cardiac Structure and Function
At Exam 1, patients displayed increased LV mass and RWT indicating concentric remodeling (Table 3). In the total population,.71% of the patients showed LV systolic dysfunction assessed by LVGLS and 34% of the patients showed RV systolic dysfunction assessed by RVFWLS (Table 3). Among the population with abdominal CT, LV mass was correlated with VAT-area (r=0.52, p<0.0001); but it was not correlated with SAT-area (r=−0.01, p=1.0) (Figure 2). RWT correlated positively with VAT-area (r=0.48, p=0.001) but did not correlate with BW or BMI. LV interventricular septal dimension and posterior wall dimension had stronger correlations with VAT-area (r=0.61, p<0.0001 and r=0.61, p<0.0001) compared with BW (r=0.36, p<0.0001 and r=0.34, p<0.0001) or BMI (r=0.20, p=0.007 and r=0.17, p=0.02) (all comparisons of R coefficient were p<0.05) (Figure 2). These findings were also confirmed restricting the analysis to include only patients with VAT assessment (Supplemental Figure 2). LVGLS correlated inversely with VAT-area (r= −0.31, p=0.047). RVGLS also correlated negatively with VAT-area (r=−0.36, p=0.04) but did not correlate with BW or BMI (Figure 2).
Table 3.
Longitudinal Changes in Left and Right Heart Structure and Function
| n | Exam 1 | Exam 2 | P-value Exam 1 vs Exam 2 |
Change from baseline | |
|---|---|---|---|---|---|
| Left Heart Structure & Function | |||||
| Septal wall thickness (mm) | 176 | 11.2±1.9 | 10.8±1.7 | 0.02 | −0.4±2.0 |
| Posterior wall thickness (mm) | 176 | 10.8±1.6 | 10.0±1.5 | <0.0001 | −0.8±1.8 |
| LV end-diastolic dimension (mm) | 185 | 50.5±5.7 | 50.4±5.9 | 0.8 | −0.1±−5.7 |
| LV end-systolic dimension (mm) | 185 | 32.0±4.8 | 32.5±5.3 | 0.1 | −0.7±5.0 |
| Relative wall thickness | 173 | 0.43±0.7 | 0.40±0.07 | <0.0001 | −0.03±0.09 |
| LV end diastolic volume (ml) | 185 | 123±33 | 123±34 | 0.9 | 0±32 |
| LV end systolic volume (ml) | 185 | 42±16 | 44±19 | 0.1 | 2±15 |
| LV mass (g) | 173 | 206 (169, 244) | 181 (154, 233) | 0.0002 | −16 (12, 53) |
| LV mass/height2.7(g/m2.7) | 173 | 49 (43, 60) | 46 (40, 56) | 0.003 | −3±13 |
| LV ejection fraction (%) | 173 | 66±7 | 64±8 | 0.003 | −2±7 |
| Endocardial fractional shortening (%) | 185 | 36.6±4.9 | 35.2±5.7 | 0.003 | −1.4±6.2 |
| Cardiac output (ml/min) | 156 | 6.9 (5.9, 7.8) | 6.3 (5.2, 7.2) | <0.0001 | −0.6±1.8 |
| Stroke volume (ml) | 156 | 98 (83, 113) | 93 (78, 107) | 0.006 | −6±25 |
| Left atrial volume (ml) | 150 | 61 (47, 79) | 69 (54, 87) | <0.0001 | 8±23 |
| Mitral annular e’ (cm/sec) | 173 | 8.1±2.3 | 7.4±2.4 | 0.002 | −0.7±2.7 |
| E/e’ ratio | 171 | 10.0 (8.2, 14.0) | 11.3 (8.6, 15.0) | 0.04 | 0.9 (−2.0, 3.2) |
| LV GLS (%) | 158 | 14.3±3.2 | 15.1±3.0 | 0.0006 | 0.8±3.0 |
| LV systolic dysfunction (%)** | 158 | 111 (71) | 94 (59) | 0.005 | −17 (−12) |
| LA strain (%) | 137 | 26.6±8.4 | 24.5±8.8 | 0.002 | −1.8±10.1 |
| Right Heart Structure & Function | |||||
| RV basal dimension (mm) | 158 | 37.8±7.0 | 37.8±7.9 | 0.1 | −1.0±7.6 |
| RV mid cavity dimension (mm) | 157 | 32.3±6.6 | 30.6±6.5 | 0.02 | −1.0±8.1 |
| RV longitudinal dimension (mm) | 157 | 78.3±9.8 | 76.4±8.7 | 0.006 | −2.5 (−6.7, 2.8) |
| RV fractional area change (%) | 142 | 41.6±7.8 | 42.4±8.5 | 0.3 | −0.8±9.9 |
| Estimated RVSP (mmHg) | 157 | 31.9±10.5 | 32.4±9.3 | 0.9 | 0.4±11.2 |
| Estimated RA pressure (mmHg) | 163 | 6.1±2.5 | 6.3±3.2 | 0.7 | 0.1±3.3 |
| RVGLS (%) | 122 | 18.2±4.8 | 19.5±4.3 | 0.002 | 1.3±6.2 |
| RVFWLS (%) | 123 | 22.2±5.8 | 24.2±5.6 | 0.009 | 2.0±8.1 |
| RV systolic dysfunction (%)** | 123 | 42 (34) | 24 (20) | 0.02 | −18 (−14) |
| Pericardial restraint | |||||
| EAT thickness (mm) | 179 | 7.4±3.8 | 5.5±3.5 | <0.0001 | −1.8±3.7 |
| Total heart volume (ml) | 167 | 865 (740, 1146) | 853 (733, 1064) | 0.1 | −40±254 |
| Biatrial volume (ml) | 167 | 223 (183, 316) | 250 (189, 334) | 0.3 | 11±112 |
| Biventricular volume (ml) | 167 | 654 (541, 819) | 616 (531, 754) | 0.0007 | −50±184 |
| Biatrial volume/total heart volume (%) | 167 | 36 (29, 45) | 39 (31, 51) | 0.0005 | 2.1±6.8 |
| Ideal/actual radius at end diastole | 157 | 1.17±0.12 | 1.12±0.09 | <0.0001 | −0.04±0.14 |
| Ideal/actual radius at end systole | 132 | 1.02±0.09 | 0.99±0.14 | 0.006 | −0.05±0.12 |
| Eccentricity index at end diastole | 157 | 1.10±0.13 | 1.02±0.11 | <0.0001 | −0.07±0.15 |
| Eccentricity index at end systole | 133 | 1.13±0.09 | 1.09±0.08 | <0.0001 | −0.03±0.11 |
IVS, inter ventricular septal; PW, posterior wall; LV, left ventricular; RWT, relative wall thickness; LA, left atrial; GLS, global longitudinal strain; RV, right ventricular; RVSP, right ventricular systolic pressure; RA, right atrial; FWLS, free wall longitudinal strain; EAT, epicardial adipose tissue.
Normal value of LVGLS is defined by LVGLS ≥16%, and that of RVFWLS is defined as RVFWLS ≥20%
Figure 2: Heatmap showing correlations between body compositions and cardiac structure/function.
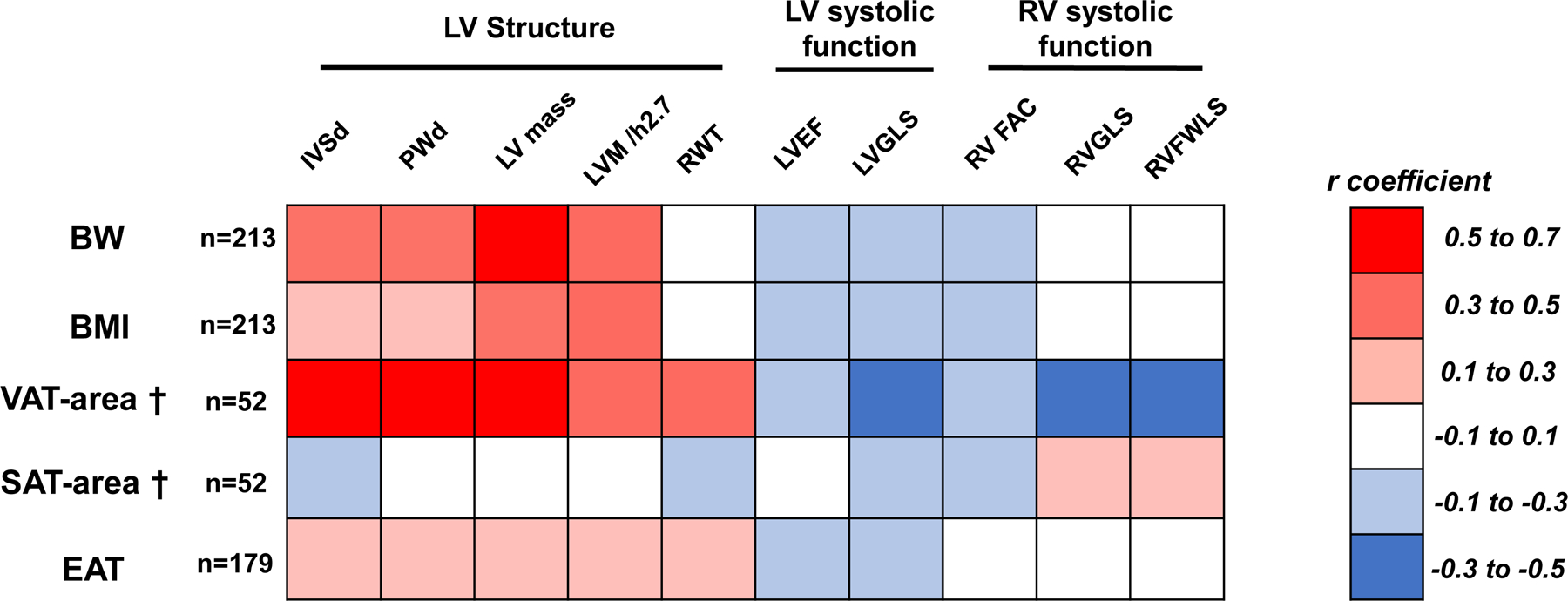
Colors show strength and direction of correlation between measures of body fat with myocardial structure and function in the study cohort. †Available for n=52. BW, body weight; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; IVSd, interventricular septal dimension; PWd, posterior wall dimension; LV, left ventricular; RWT, relative wall thickness; EF, ejection fraction; GLS, global longitudinal strain; RV, right ventricular; FAC, fractional area change; FWLS, free wall longitudinal strain.
Effects of Weight Loss on Body Composition, Metabolism, and Comorbidities
The median duration between Exam 1 and Exam 2 in the study population was 5.3 (interquartile range 2.9, 7.9) years. Follow up duration was not significantly different between the subgroups with and without abdominal CT (4.5 [IQR 3.0–7.5] vs. 5.4 [IQR 2.9–8.1] years, p=0.5). BW was reduced by 23%, and BMI showed a mean 22% reduction overall (Table 3, Figure 3A). In the subgroup of patients with abdominal CT, both VAT-area and SAT-area significantly decreased by 30% and 29% from the baseline. Among the overall population, epicardial adipose tissue thickness was reduced by 14%. Estimated plasma volume decreased in tandem with the WL.
Figure 3: Effects of Weight Loss on Cardiac Structure and Function.
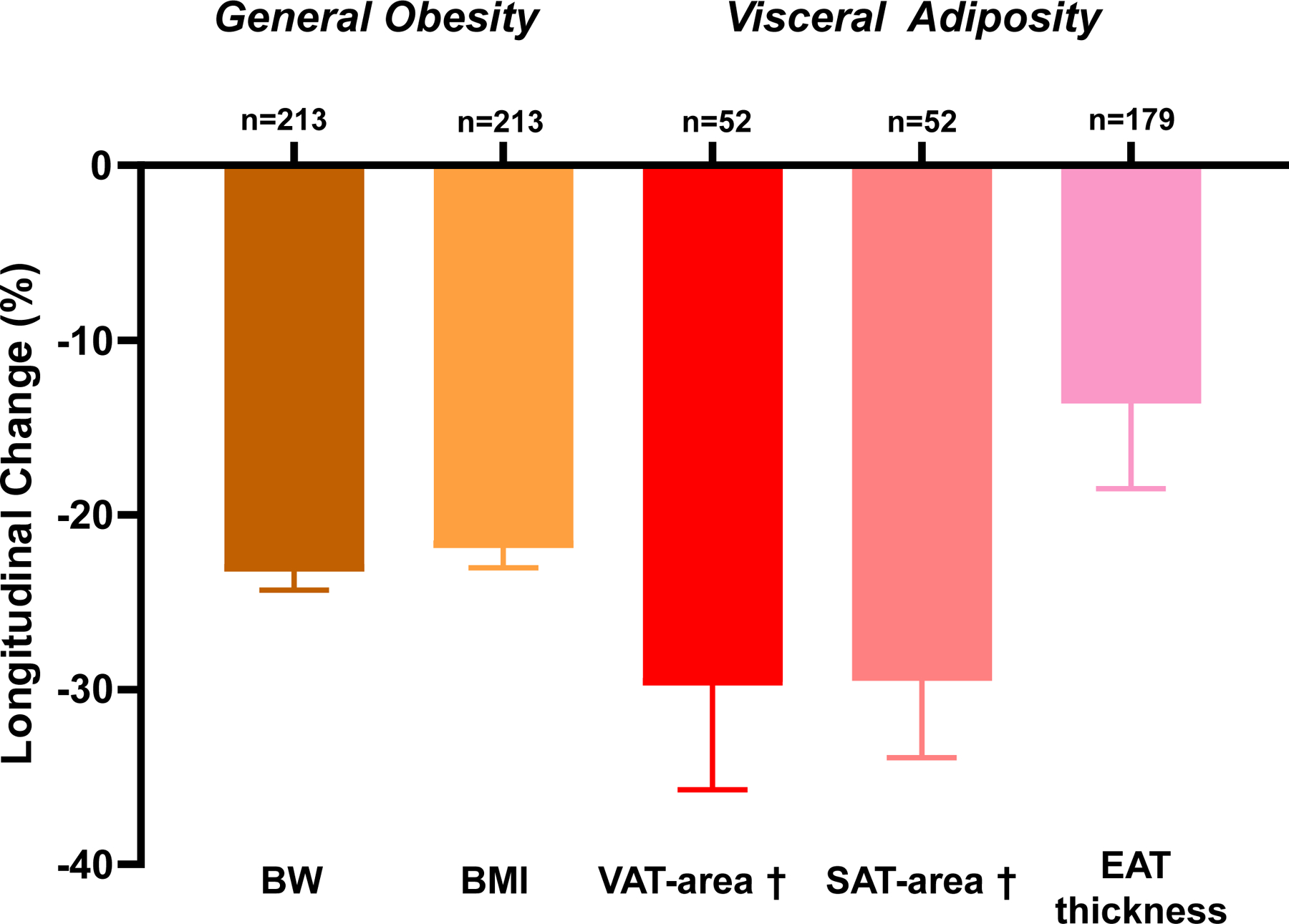
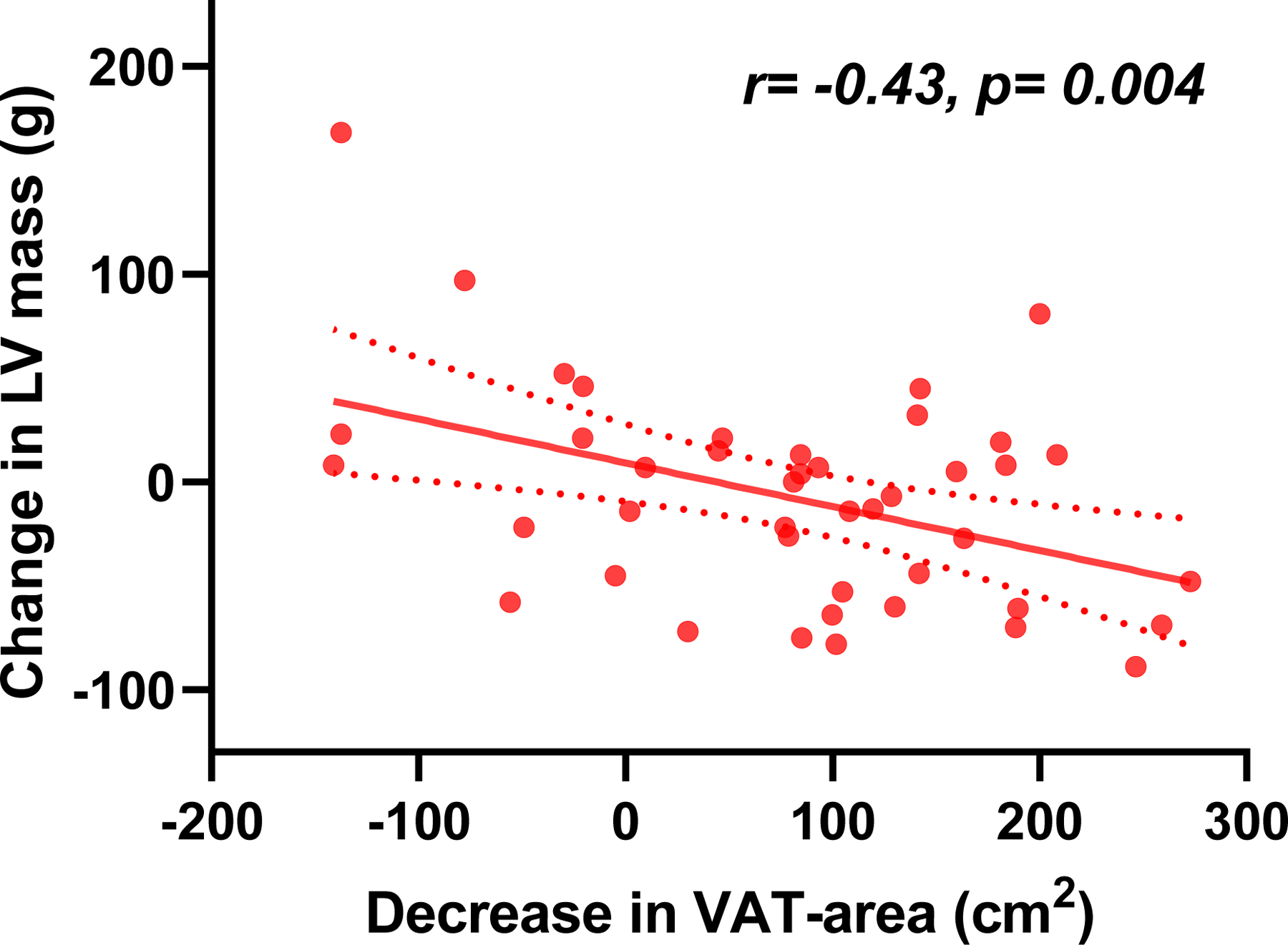
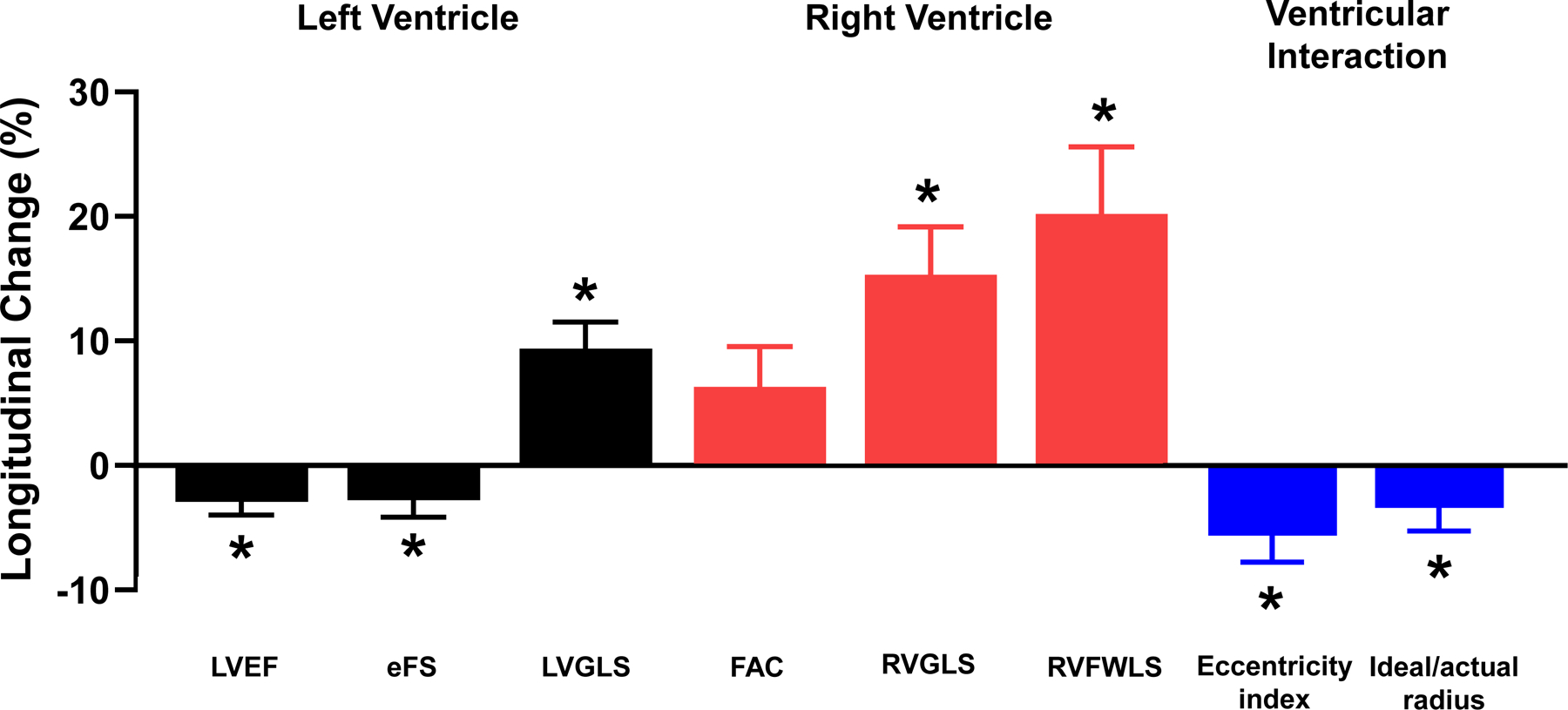
(A) Changes in body mass components. (B) Changes in LV mass were modestly correlated with changes in VAT-area. (C) Reduction in LVEF and eFS and improvements in LV GLS were observed. RV FAC did not change, but RV GLS and RV FWLS improved. Ventricular interaction assessed by LV eccentricity index and ideal-to-actual LV ratio was reduced following surgical weight loss. VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BW, body weight; BMI, body mass index; EAT, epicardial adipose tissue; LV, left ventricular; EF, ejection fraction; eFS, endocardial fractional shortening; FAC, fractional area change; GLS, global longitudinal strain; RV, right ventricular; FWLS, free wall longitudinal strain. Eccentricity index and Ideal/actual radius were measured at end diastole. *p<0.05 between Exam 1 and Exam 2. †Available for n=52. Error bars indicate SEM in Figure 3A and 3C. Solid line represents regression line and dashed lines represent 95% confidence interval in Figure 3B
A decrease in the levels of fasting glucose and HbA1c were observed, suggesting improved insulin sensitivity. Heart rate and blood pressure decreased, despite no change in use of antihypertensive medicines, and a decrease in diuretic usage from Exam 1 to Exam 2, mirroring the decrease in estimated plasma volume (Table 3). The prevalences of diabetes and obstructive sleep apnea (OSA) decreased following surgery, but the prevalences of both atrial fibrillation and HFpEF both increased, despite marked weight reduction (Table 2).
Longitudinal Changes in the LV following Weight Loss
LV mass decreased because of a reduction in the LV wall thickness, without a change in the LV diastolic dimension, and both cardiac output and stroke volume decreased following WL in tandem with the reduction in body weight and estimated plasma volume (Table 3). Changes in LV mass were correlated with change in VAT-area (r=−0.43, p=0.004) (Figure 3B), but were not correlated with changes in SAT-area (r=0.05, p=0.8) or with changes in EAT (r=0.06, p=0.4).
Modest but significant reductions in systolic LV endocardial chamber function (LVEF and eFS) were observed, but LV tissue mechanics improved following WL, evidenced by an increase in LVGLS (Table 3, Figure 3C).
Changes in the RV and Ventricular Interaction following Weight Loss
RV mid-cavity and longitudinal dimensions significantly decreased following surgery (Table 3). Analogous to changes in the LV, RVGLS and RVFWLS both improved following WL, without a significant change in RVFAC (Figure 3C). WL was not associated with changes in estimated RV systolic pressure or estimated right atrial pressure.
The reduction in EAT was coupled with significant decreases in measures of pericardial restraint and ventricular interdependence, including ideal/actual radius and LV eccentricity index (Table 3, Figure 3C). While total heart volume did not change significantly, biventricular volume decreased and the ratio of biatrial volume/total heart volume increased.
Changes in estimated LA Pressure, Structure and Function
Despite favorable changes in biventricular remodeling and systolic mechanics following surgical WL, at the median follow up of 5.3 years, LA volume had increased, LA reservoir strain decreased, and LV filling pressures estimated by the E/e’ ratio increased (Table 3). Time-dependent impairments in LA reservoir strain were similar in patients who did or did not develop atrial fibrillation between examinations.
Increases in LA volume were found to be greater in those with follow up duration greater than the median (+11±23 mL vs +4±22 mL, p=0.049), with similar findings observed for E/e’ (+1.5[−1.7, 3.9] vs −0.4[−2.7, 2.5], p=0.04) (Supplemental Figure 4), suggesting that part of the increase is related to time-dependent progression in LA myopathy associated with aging. In univariate linear regression analysis, there were significant associations between changes in LA volume (β (95% confidence interval [CI]) =1.19 (0.04, 2.33), p=0.04); and E/e’ ratio (β (95% CI) =0.25 (0.01, 0.50), p=0.04) with the duration between examinations, whereas other changes in cardiac structure/function were not related to the time interval between examinations (Supplemental Table 3), supporting this potential explanation.
LA volume enlarged and E/e’ increased more in patients with new onset heart failure compared to those without (Supplemental Table 4), but there were no significant differences among those with new onset of atrial fibrillation (Supplemental Table 5).
DISCUSSION
In this study, we aimed to characterize the impact of bariatric surgery on fat distribution and changes in the heart in patients with medically-complicated obesity over a longer-term follow up than previously reported, with a focus on the relationships with visceral fat reduction in the abdomen and epicardial space (Central Illustration). In agreement with prior studies, LV reverse remodeling was observed following bariatric surgery in the entire population, and here we show these changes were observed to be most strongly tied to reductions in visceral fat rather than subcutaneous fat or body weight in the smaller subgroup of patients with abdominal CT imaging obtained. Favorable changes in ventricular structure were coupled with modest reduction in endocardial functional indices, but improvements in LV and RV systolic mechanics, assessed by LVGLS, RVGLS and RVFWLS. Decreases in LV mass and RV size were coupled with significant reductions in epicardial fat, leading to decreases in pericardial restraint and ventricular interaction. Despite favorable effects on biventricular remodeling and mechanics, there was progression in LA remodeling and worsening LA dysfunction, with increasing prevalence in atrial fibrillation, and greater elevation filling pressures, at least as estimated by E/e’ ratio. These data suggest that while WL is beneficial for many of the cardiac changes that lead to HFpEF, the risk is not fully reduced over time, despite marked weight loss. These data underline the priority for randomized controlled trials testing the effects of WL on cardiac function and clinical outcomes in patients with or at risk for HFpEF.
Central Illustration:
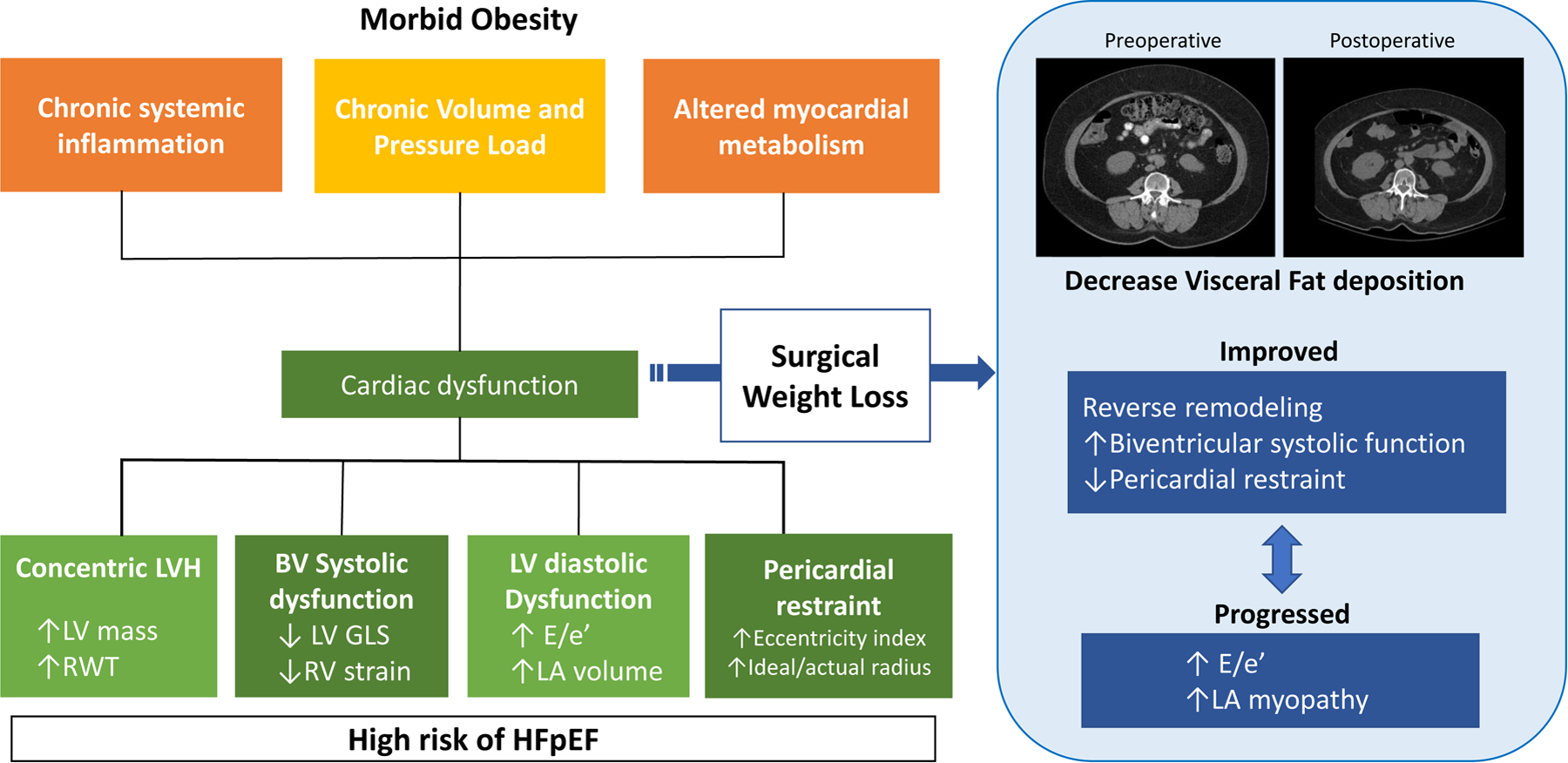
Cardiac dysfunction in Obesity and Effects of Surgical Weight Loss. Systemic inflammation, volume overload and derangement of myocardial metabolism caused by morbid obesity result in myocardial dysfunction. Surgical weight loss improved LV remodeling, biventricular function, and pericardial restraint. In contrast, elevation of E/e’ and LA myopathy progressed.
Visceral Fat and Cardiovascular Structure and Function
In cross sectional analyses, we observed that LV remodeling and dysfunction was more strongly correlated with visceral adiposity rather than subcutaneous fat, or with general obesity as measured by BW or BMI. Obesity, especially visceral adiposity, is recognized as a central player of cardiac dysfunction related to HF.3, 4 Visceral adipose secretes pro-inflammatory adipokines, leading to a chronic low-grade systemic inflammation,19 as well as pro-fibrotic mediators that may contribute to remodeling and diastolic dysfunction.20 Excess visceral adipose may promote plasma volume expansion through the activation of neurohormones.21 The fact that LV wall thickness and RVGLS were more strongly correlated with VAT than BW or BMI supports a relatively stronger impact of visceral adiposity on cardiac dysfunction in morbid obesity, just as recently identified in HFpEF.4
We observed a 20–25% reduction in BW following bariatric surgery; but VAT-area decreased (numerically) to greater extent (by 30% reduction). Changes in LV mass were correlated with changes in VAT-area (r=−0.43, p=0.004) (Figure 2B) but not SAT-area (r=0.05, p=0.8), again pointing to a more important role for visceral fat reduction in obesity-related heart disease. Further study is required to determine whether and how reductions in VAT might have a causal role in the observed changes in cardiac structure and function in obesity.
Improvement in LV Mechanics following Weight Loss
In patients with preserved EF, WL has been associated with variable effects on LV systolic function measured at the endocardium, with results ranging from benefit, to no change, to slight impairment.11, 13 In the present study, we observed an improvement in LVGLS, in agreement with a few small series with shorter follow up durations,22–24 but this occurred despite modest reductions in endocardial function (LVEF and eFS).
While the disagreement between effects on myocardial deformation (GLS) and endocardial function may seem perplexing or even concerning at first blush, this finding may be predictable based upon the confounding effects of LV remodeling on endocardial function. Stokke et al. demonstrated that increases in wall thickness allow for preservation of EF despite impairments in myocardial deformation, essentially concealing systolic dysfunction in such patients.25 This mirrors what is seen in HFpEF as well, where there are abnormalities in midwall myocardial shortening and GLS, despite normal EF, in relation to increases in concentric remodeling.26, 27
The present results are consistent with this relationship in patients with obesity, most of whom did not have frank HFpEF, as it appears that the modest reductions in EF and eFS observed following surgery are related to reverse remodeling rather than an impairment in systolic function. This is consistent with findings from Owan et al., who observed striking improvements in midwall fractional shortening 2 years following bariatric surgery, despite no change in EF.10 Midwall shortening is determined by the contraction of muscle fibers oriented in orthogonal directions at the inner and outer surfaces of the heart, and is thus highly sensitive to impairments in longitudinal systolic function, which are better reflected by GLS.25, 26
Improvement in RV Mechanics following Weight Loss
RV dysfunction is associated with poor prognosis in patients with HFpEF,28, 29 and patients with obesity related HFpEF display more severe RV remodeling and dysfunction compared to non-obese patients.6 Mirroring the LV, RV strain correlated negatively with VAT-area but did not correlate with BW or BMI (Figure 1), and both RVGLS and RVFWLS improved after bariatric surgery (Figure 2C). These findings suggest that WL, particularly reduction in visceral adiposity, benefit RV structure and function in a manner akin to that observed in the LV. One pertinent neutral finding relates to the absence of an effect on RV afterload, estimated by RVSP, which did not change following WL. This finding disagrees with a prior meta-analysis of invasive studies.30 The reason for the discrepancy is not clear, but may relate to the use of echocardiography to estimate hemodynamics, as recent data indicate that relationships between echocardiographic hemodynamic estimates and directly measured values differ between patients with and without excess body fat.31
The mechanism of benefit for LV and RV systolic mechanics following WL cannot be determined from the present study, but obesity is known to increase myocardial oxygen demand and impair efficiency,32 and prior studies have shown improvements in myocardial metabolism and energy utilization following WL, which may contribute.7 Favorable changes in blood pressure and plasma volume may certainly contribute. Obesity is also associated with myocardial steatosis, which has been correlated with impairments in GLS in patients with diabetes but no HF33 and may be reduced through long-term WL.34
Reductions in Epicardial Fat and Ventricular Interaction
Patients with HFpEF and increased EAT display greater elevation in cardiac filling pressures, more severe pulmonary hypertension, amplified pericardial restraint, poorer exercise capacity, and as recently shown, increased risk for adverse outcomes.6, 16, 35–37 Increases in EAT have also been strongly tied to abnormalities in myocardial deformation and fibrosis in patients without HF.38, 39 Excessive EAT has been proposed as a potential source of inflammatory adipokines that may influence myocardial function and contribute to HFpEF.40
The present study revealed a decrease in EAT, mirroring changes in abdominal VAT. The reduction in EAT following bariatric surgery was proportionately less than abdominal VAT, in agreement with an earlier study,41 but the pathophysiologic significance for even minor reductions in EAT may be substantial for a number of reasons. In addition to soluble effects related to adipokines, increases in EAT and epicardial heart volume amplify coupling between the right and left sides of the heart, a phenomenon known as ventricular interdependence.6, 42 Here we show for the first time that WL induced through bariatric surgery may reduce this pericardial restraint and ventricular interdependence, which would be expected to reduce cardiac filling pressures and relieve lung congestion.31, 42
The observed increase in E/e’ over time was surprising in light of prior invasive studies showing favorable reductions in directly-measured cardiac filling pressures following WL.30 However, it may be that E/e’ is not a reliable estimate of LV filling pressures in this setting, or that its relationship with directly measured LA pressure differs with body mass.31 Indeed, an increase in extrinsic restraint on the heart (as with increased EAT) reduces the transmural distending pressure relative to measured intracavitary pressure. Surgical WL may shift in this relationship, such that a higher E/e’ is actually reflective of the same or even lower filling pressure.31
Uncoupling of Atrial and Ventricular Effects
Despite improvements in ventricular remodeling, function, and pericardial restraint following WL, LA structure and function deteriorated, with an increase in LA volume, decrease in LA reservoir strain, an elevation in estimated LA pressures, and an increase in the prevalence of AF. In obese patients, LA volume is positively related to LV mass,43 suggesting that WL should reduce LA volume, but here we show that LA enlargement progresses in obesity-related heart disease over longer durations of follow-up, despite beneficial reverse LV remodelling that occurs early and is maintained. This may partly reflect the effects of time, as prior studies in community-based samples indicate that LA volume and E/e’ ratio both increase as part of normal aging,44 but the disconnect between atrial and ventricular effects is nonetheless notable and suggests a fundamental difference. A recent meta-analysis of observational studies demonstrated a highly significant 50% reduction in the incidence of new-onset HF following bariatric surgery, but there was no significant effect on incident AF.9 The present results revealing progression of LA myopathy, despite of favorable effects at the level of ventricles, may help explain this relatively less marked improvement in AF reduction following WL. However, these results are difficult to interpret because of the lack of a control group of patients who did not undergo WL surgery. This further points to the importance of prospective trials.
Limitations
This is a retrospective observational study and restricted to patients referred for and who underwent bariatric surgery, all of which introduces bias. There was no separate control group of patients with obesity but no WL intervention, precluding ability to make inferences regarding causality. Multiple metabolic, physical, and loading changes occur following bariatric surgery, including improvements in insulin sensitivity, reductions in blood pressure and lipids, and decrease in obesity-related comorbidities such as sleep apnea, and the present analysis cannot determine the extent to which observed changes in cardiac structure and function were related to WL alone or to these coexisting changes. These limitations emphasize the importance of performing rigorous randomized controlled trials testing WL either through surgical, lifestyle or pharmacologic interventions. Some such trials are already underway, evaluating the effects of pharmacologic WL induced by GLP-1 receptor agonists and combined GLP-1/GIP agonists (NCT04788511, NCT04847557) on clinical endpoints such as quality of life and physical function. The retrospective nature of the study restricts the sample to patients who underwent necessary imaging, leading to selection bias. In addition, the important variables supporting the associations of cardiac structure and function with visceral adiposity were derived from a smaller subgroup including 25% of patients. While this data missingness could create bias, baseline characteristics were similar in the subgroup with VAT data (Supplemental Table 2), overall relationships between body weight and cardiac structure/function were similar to the broader sample (Supplemental Figures 2, 3), and it seems less likely a priori that referral for abdominal imaging would influence the relationship between VAT or VAT changes and cardiac structure/function.
Conclusions
In this observational study, weight loss following bariatric surgery was observed to be associated with LV reverse remodeling, improved longitudinal biventricular mechanics, and reduced ventricular interaction, and these benefits seemed to be related with reductions in abdominal and epicardial visceral fat after long-term follow up. Despite beneficial effects in the ventricles, LA structure and function deteriorated over time, which may contribute to the less marked reduction in AF noted following bariatric surgery. These data provide new insights into the effect of WL on cardiac dysfunction in obesity-related heart disease and emphasize the priority for future controlled trials testing the effects of surgical, pharmacologic and lifestyle WL interventions on the heart and clinical outcomes in patients with or at risk for obesity-related HFpEF
Supplementary Material
Clinical Perspectives.
Clinical Competency:
This study shows that weight loss following bariatric surgery is associated with improvements in ventricular structure and function, and reductions in ventricular interdependence, which are closely tied to decreases in visceral and epicardial fat.
Translational Outlook:
These data emphasize the need for carrying out prospective, randomized trials of weight loss as a treatment for obesity-related HFpEF.
Funding:
Dr. Borlaug was supported by R01 HL128526 and U01 HL 160226, both from the National Institutes of Health (NIH). H.S. is supported by a research fellowship from the Uehara Memorial Foundation, Japan. Dr Rider was supported by BHF Intermediate Clinical Fellowship FS/16/70/32157.
Abbreviations List
- BMI
body mass index
- BW
body weight
- EAT
epicardial adipose tissue
- eFS
endocardial fractional shortening
- FWLS
free wall longitudinal strain
- GLS
global longitudinal strain
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- LV
left ventricular
- OSA
obstructive sleep apnea
- RV
right ventricular
- RWT
relative wall thickness
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
- WL
weight loss
Footnotes
Disclosures: BAB has received research grants from NIH/NHLBI, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, Tenax Therapeutics and has received consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. OJR has received consulting fees from Amgen, Cytokinetics, Servier and GSK. The remaining authors have nothing to disclose.
References
- 1.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW and Gortmaker SL. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM and Vasan RS. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao VN, Fudim M, Mentz RJ, Michos ED and Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, Koepp KE, Khosla S, Jensen MD and Borlaug BA. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J 2021;42:1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, Takahashi N, Sunagawa K and Borlaug BA. Obesity, Venous Capacitance, and Venous Compliance in Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail 2021. [DOI] [PubMed]
- 6.Obokata M, Reddy YN, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayner JJ, Peterzan MA, Watson WD, Clarke WT, Neubauer S, Rodgers CT and Rider OJ. Myocardial Energetics in Obesity: Enhanced ATP Delivery Through Creatine Kinase With Blunted Stress Response. Circulation 2020;141:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J and Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Veldhuisen SL, Gorter TM, van Woerden G, de Boer RA, Rienstra M, Hazebroek EJ and van Veldhuisen DJ. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J 2022. [DOI] [PMC free article] [PubMed]
- 10.Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC and Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol 2011;57:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T and Ashrafian H. The Effects of Bariatric Surgery on Cardiac Structure and Function: a Systematic Review of Cardiac Imaging Outcomes. Obes Surg 2016;26:1030–40. [DOI] [PubMed] [Google Scholar]
- 12.Rider OJ, Francis JM, Ali MK, Petersen SE, Robinson M, Robson MD, Byrne JP, Clarke K and Neubauer S. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol 2009;54:718–26. [DOI] [PubMed] [Google Scholar]
- 13.Cuspidi C, Rescaldani M, Tadic M, Sala C and Grassi G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis. Am J Hypertens 2014;27:146–56. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 15.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D’Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD and Voigt JU. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- 16.Koepp KE, Obokata M, Reddy YNV, Olson TP and Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy YNV, Obokata M, Verbrugge FH, Lin G and Borlaug BA. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. J Am Coll Cardiol 2020;76:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi N, Sugimoto M, Psutka SP, Chen B, Moynagh MR and Carter RE. Validation study of a new semi-automated software program for CT body composition analysis. Abdom Radiol (NY) 2017;42:2369–2375. [DOI] [PubMed] [Google Scholar]
- 19.Fontana L, Eagon JC, Trujillo ME, Scherer PE and Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–3. [DOI] [PubMed] [Google Scholar]
- 20.Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, Marcelin G, Surenaud M, Marcos E, Gual P, Clement K, Hue S, Adnot S, Hatem SN, Tsuchimochi I, Yoshimitsu T, Henegar C and Derumeaux G. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018;138:809–822. [DOI] [PubMed] [Google Scholar]
- 21.Packer M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People With Obesity. Circulation 2018;137:1614–1631. [DOI] [PubMed] [Google Scholar]
- 22.Kaier TE, Morgan D, Grapsa J, Demir OM, Paschou SA, Sundar S, Hakky S, Purkayastha S, Connolly S, Fox KF, Ahmed A, Cousins J and Nihoyannopoulos P. Ventricular remodelling post-bariatric surgery: is the type of surgery relevant? A prospective study with 3D speckle tracking. Eur Heart J Cardiovasc Imaging 2014;15:1256–62. [DOI] [PubMed] [Google Scholar]
- 23.Tuluce K, Kara C, Tuluce SY, Cetin N, Topaloglu C, Bozkaya YT, Saklamaz A, Cinar CS and Ergene O. Early Reverse Cardiac Remodeling Effect of Laparoscopic Sleeve Gastrectomy. Obes Surg 2017;27:364–375. [DOI] [PubMed] [Google Scholar]
- 24.Shin SH, Lee YJ, Heo YS, Park SD, Kwon SW, Woo SI, Kim DH, Park KS and Kwan J. Beneficial Effects of Bariatric Surgery on Cardiac Structure and Function in Obesity. Obes Surg 2017;27:620–625. [DOI] [PubMed] [Google Scholar]
- 25.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T and Remme EW. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. J Am Coll Cardiol 2017;70:942–954. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ and Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009;54:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA and Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melenovsky V, Hwang SJ, Lin G, Redfield MM and Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obokata M, Reddy YNV, Melenovsky V, Pislaru S and Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy YNV, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE and Borlaug BA. Hemodynamic Effects of Weight Loss in Obesity: A Systematic Review and Meta-Analysis. JACC Heart Fail 2019;7:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obokata M, Reddy YNV, Melenovsky V, Sorimachi H, Jarolim P and Borlaug BA. Uncoupling between intravascular and distending pressures leads to underestimation of circulatory congestion in obesity. Eur J Heart Fail 2021. [DOI] [PubMed]
- 32.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T and Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–6. [DOI] [PubMed] [Google Scholar]
- 33.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ and Bax JJ. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010;122:2538–44. [DOI] [PubMed] [Google Scholar]
- 34.Abdesselam I, Dutour A, Kober F, Ancel P, Bege T, Darmon P, Lesavre N, Bernard M and Gaborit B. Time Course of Change in Ectopic Fat Stores After Bariatric Surgery. J Am Coll Cardiol 2016;67:117–9. [DOI] [PubMed] [Google Scholar]
- 35.van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, Rienstra M, Westenbrink BD and Gorter TM. Epicardial Adipose Tissue and Outcome in Heart Failure With Mid-Range and Preserved Ejection Fraction. Circ Heart Fail 2021:CIRCHEARTFAILURE121009238. [DOI] [PMC free article] [PubMed]
- 36.Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, Hoendermis ES and van Veldhuisen DJ. Epicardial Adipose Tissue and Invasive Hemodynamics in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8:667–676. [DOI] [PubMed] [Google Scholar]
- 37.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, Flammer A, Borlaug BA, Ruschitzka F and Masi S. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–1871. [DOI] [PubMed] [Google Scholar]
- 38.Ng ACT, Strudwick M, van der Geest RJ, Ng ACC, Gillinder L, Goo SY, Cowin G, Delgado V, Wang WYS and Bax JJ. Impact of Epicardial Adipose Tissue, Left Ventricular Myocardial Fat Content, and Interstitial Fibrosis on Myocardial Contractile Function. Circ Cardiovasc Imaging 2018;11:e007372. [DOI] [PubMed] [Google Scholar]
- 39.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K and Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2015;36:795–805a. [DOI] [PubMed] [Google Scholar]
- 40.Packer M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J Am Coll Cardiol 2018;71:2360–2372. [DOI] [PubMed] [Google Scholar]
- 41.Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clement K, Bernard M and Dutour A. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol 2012;60:1381–9. [DOI] [PubMed] [Google Scholar]
- 42.Borlaug BA and Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottdiener JS, Reda DJ, Williams DW and Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol 1997;29:651–8. [DOI] [PubMed] [Google Scholar]
- 44.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ and Rodeheffer RJ. Longitudinal Changes in Left Ventricular Stiffness: A Community-Based Study. Circ Heart Fail 2013;6:944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


