Fig. 1: PKG activity in heart extracts and phosphoproteomic anaylsis of cardiac myocytes isolated from Prkg1RQ/+ mice and control litter mates.
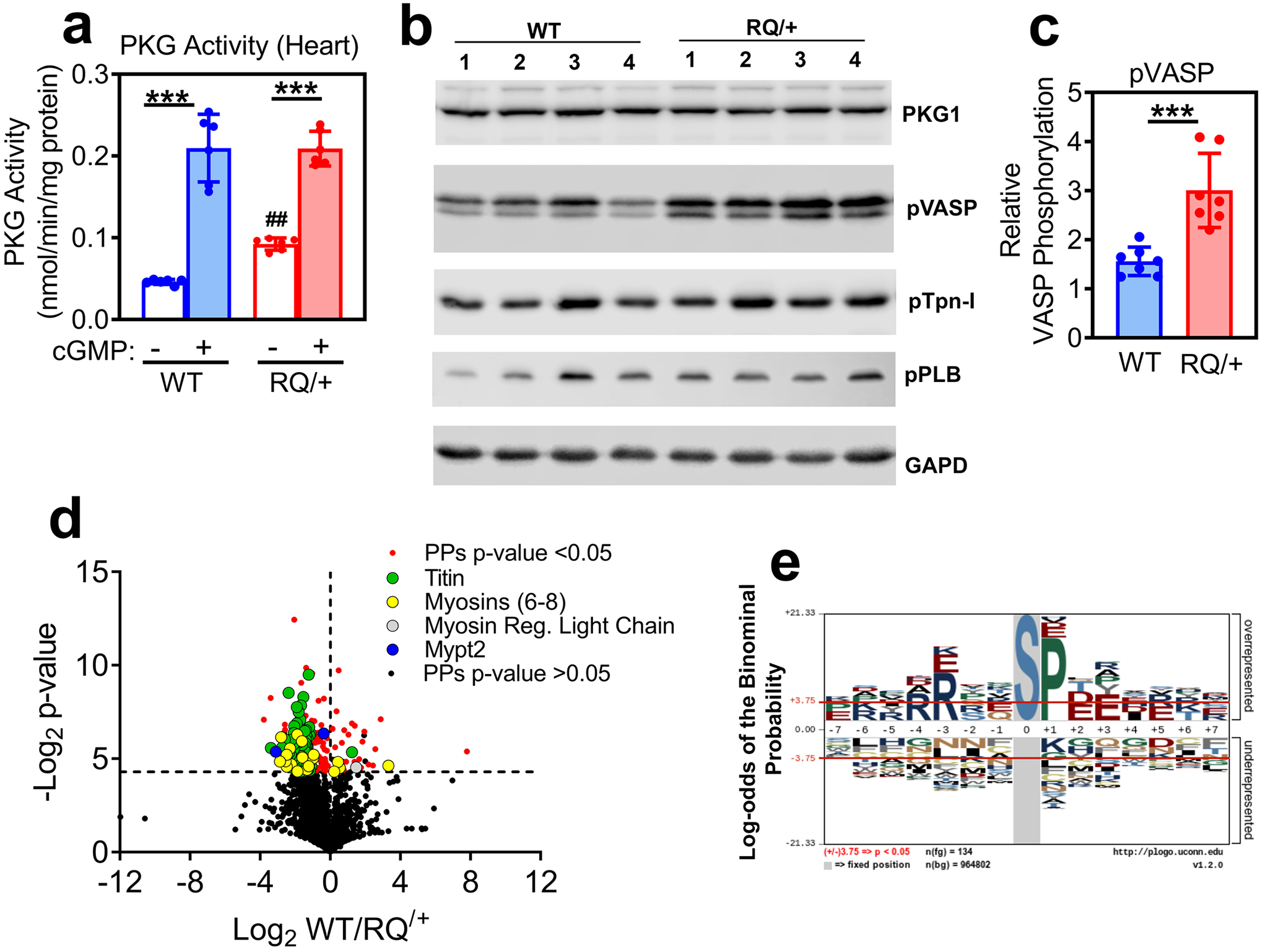
(a) PKG activity was measured in heart extracts from wild type (WT) and Prkg1RQ/+ (RQ/+) mice using an optimized peptide substrate in the absence and presence of cGMP (n=6 mice per genotype; means +/− SD, ***p<0.001 for comparison +/− cGMP and ##p<0.01 for comparison to wild type). (b) Western blots of heart extracts from four wild type and four Prkg1RQ/+ mice were developed with antibodies specific for PKG1, Ser239-phospho-VASP (a preferred PKG substrate site), Ser23/24-phosphorylated troponin I (pTpn-I), Ser16/Thr17-phosphorylated phospholamban (pPLB), or GAPD (loading control). (c) VASP phosphorylation (assessed as in panel b) was quantified using a Li-COR Odyssey Scanner, and normalized to GAPD (n=7 mice per genotype, ***p<0.001). (d) The phosphoproteome of adult cardiac myocytes freshly isolated from WT and RQ/+ mice (n=4 per genotype) was analyzed, and the Log2 differences between WT and RQ/+ were plotted over Log2 t-test values (Volcano plot) for all peptides identified at least once in every sample. Significantly different phospho-peptides (PPs, −Log2 t-test p-values >4.3) are shown in red, with peptides from some sarcomeric proteins highlighted in green (titin), yellow (myosin 6, 7, 8), grey (myosin regulatory light chain) and blue (Mypt2). (e) A graphical representation of the most frequently encountered serine (S)-centered phosphorylation consensus motifs is shown for cardiac myocytes from RQ/+ mice.
