Abstract
Background:
High dosage opioid use is a risk factor for opioid-related overdose commonly cited in guidelines, recommendations, and policies. In 2012, the Oregon Medicaid program developed a prior authorization policy for opioid prescriptions above 120 mg per day morphine equivalent dose (MED). This study aimed to evaluate the effects of that policy on utilization, prescribing patterns, and health outcomes.
Methods:
Using administrative claims data from Oregon and a control state (Colorado) between 2011 and 2013, difference-in-differences analyses were used to examine changes in utilization, measures of high risk opioid use, and overdose after introduction of the policy. Opioid utilization in a cohort of individuals who were high dosage opioid users before the policy was also evaluated.
Results:
Following implementation of Oregon’s high dosage policy, the monthly probability of an opioid fill over 120 mg MED declined significantly by 1.7 percentage points (95% confidence interval [CI]; −2.0% to −1.4%), whereas it increased significantly by 1.0 percentage points (95% CI 0.4% to 1.7%) for opioid fills < 61 mg MED. Fills of medications used to treat neuropathic pain also increased by 1.2 percentage points (95% CI 0.7% to 1.8%). The monthly probability of multiple pharmacy use declined by 0.1 percentage points (−0.2% to −0.0) following the prior authorization, but there were no significant changes in ED encounters or hospitalizations for opioid overdose. Among individuals who were using a high dosage opioid before the policy, there was a 20.3 percentage point (95% CI −15.3% to −25.3%) decline in estimated probability of having a high dosage fill after the policy.
Conclusions:
Oregon’s prior authorization policy was effective at reducing high dosage opioid prescriptions. While multiple pharmacy use also declined, no changes in opioid overdose were observed.
Keywords: Medicaid, opioid, overdose, prior authorization
Introduction
The prescription opioid overdose epidemic is a public health crisis. Mortality from prescription opioid overdose quadrupled* between 1999 and 2014, increasing from 1.2 to 4.6 per 100,000 persons.1 Opioid overdoses are closely associated with medication duration of action,2 chronicity of use,3 concurrent benzodiazepine use, and using higher doses.4 The association with opioid dosage is particularly strong, with several studies demonstrating a dose-related overdose risk.5–13
Many organizations have issued prescribing guidelines recommending clinicians be vigilant when prescribing opioids in high dosages.14 In 2007, the state of Washington developed some of the first clinical guidelines that recommended clinicians be cautious prescribing opioids at dosages at or above 120 mg per day morphine equivalent dose (MED).15 Following dissemination of these guidelines, the Washington Medicaid program reported significant reductions in the use of opioid above the 75th percentile in daily dosage, but no change to the median overall dosage.16
In addition to guidelines, health care payers have tools available to manage pharmacy utilization, including preferred drug lists and prior authorization. Prior authorization policies require that specific criteria be met before reimbursement and are often considered the most potent management levers available to state Medicaid programs.17 Although they are used commonly, research demonstrating their utility in managing prescription opioids is limited. A multistate study examining the prevalence and effectiveness of prior authorization for branded controlled-release oxycodone (OxyContin) across state Medicaid programs found wide variation in effect that ranged from a 76% decrease in utilization to a 9% increase.18 The Massachusetts Medicaid program added prior authorization requirements to high dosage of controlled-release oxycodone (>240 mg per day), transdermal fentanyl (200 μg per day), extended-release morphine (>360 mg per day), and methadone (>120 mg per day) in 2002, resulting in modest dosage reductions for morphine and methadone, but increases in the average dosage of both oxycodone and fentanyl during the same period.19 These recent studies did not examine changes in opioid-related adverse events.
In 2012, the Oregon Medicaid program implemented prior authorization criteria for opioid prescriptions written above 120 mg per day MED in its fee-for-service program.20 The policy was implemented in April and June of 2012 for long- and short-acting opioids, respectively. Patients with a cancer diagnosis or in a hospice program were exempt from the policy. Patients exceeding the dosage threshold were allowed up to 60 days to taper. Patients approved to remain on a high dosage required reauthorization every 6 months. Additional details about the policy are provided in the Online Supplement. Combination products were not affected because a separate and preceding policy limiting the total daily dosage of acetaminophen (<4 g per day) effectively limited the opioid dosage as well. The objective of this study was to characterize the effect of Oregon’s high dosage prior authorization policy on prescription opioid utilization, potential high-risk opioid use, and opioid-related adverse events.
Methods
Data sources and study overview
We used Medicaid administrative claims and enrollment data from the fee-for-service programs in Oregon and Colorado from January 2011 to December 2013 in all analyses. The Colorado Medicaid program, which did not have any dosage limit policies in place during this time frame, was selected as a control group because the investigators had worked previously with administrators and researchers in that state.21
For our primary analyses, we employed a difference-in-differences approach to evaluate the effect of the policy on prescription drug utilization, high-risk use, and adverse outcomes. We also conducted 2 secondary analyses. First, rather than analyzing individual/month-level utilization, we examined changes in an individual’s likelihood of filling a high dosage opioid at any point before and after the policy. Second, we ascertained the effect of the policy on a cohort of pre-implementation high dosage opioid users.
Study sample
For the primary analysis, we included individuals who met the following criteria: (1) enrolled in either the Oregon or Colorado fee-for-service Medicaid program between January 2011 to December 2013; (2) had at least 1 opioid prescription filled during this time period; and (3) were not dual Medicare/Medicaid eligible. We excluded dual-eligible recipients because of the potential for missing data collected by the Medicare program. For each individual, we created monthly observations to characterize changes in several different utilization measurements before and after the policy. There were no restrictions with respect to enrollment, and observations were censored if patients were not enrolled during the month. The secondary analysis of any high dosage opioid use included the same sample.
The sample for our cohort analysis was derived from a subset of individuals in the primary analysis. The cohort was defined by individuals enrolled at least 75% of the study period and who had 1 or more high dosage opioid prescription (>120 mg per day MED) before the policy was implemented.
Outcomes
Our primary study outcomes included total opioid prescriptions, prescriptions over 120 mg per day MED, prescriptions between 61 and 120 mg per day MED, and those less than 61 mg per day MED. MED calculations were based on established conversion factors and are summarized in Online Supplement eTable 122 We also examined utilization of drugs for arthritis, drugs used to treat neuropathic pain, and benzodiazepines to evaluate potential substitution effects. These medications are summarized in Online Supplement eTable 2.
In addition to total opioid prescription use, we also examined the following measures of high-risk opioid use23: opioid overlap, opioid benzodiazepine overlap, nonbenzodiazepine sedative hypnotic (e.g., zolpidem) use of at least 7 days, long-acting opioid for acute pain condition (defined as fill within 30 days of a medical encounter for an acute pain condition as as defined in Online Supplement eTable 3), and multiple pharmacy use. Medication overlap was defined as having at least 2 prescriptions with at least 7 days of overlap. Multiple pharmacy use was defined as having 3 or more opioid prescriptions that overlapped on at least 1 day from 3 or more pharmacies.24
Finally, we evaluated the effect of the policy on emergency department (ED) encounters and hospitalizations for opioid overdose. Opioid-related ED visits or hospitalizations were defined by the presence of the following International Classification of Disease 9th Revision Clinical Modification (ICD-9-CM) codes: poisoning by opiates and related narcotics (9650), poisoning by opium (alkaloids), unspecified (96500), poisoning by heroin (96501), poisoning by methadone (96502), poisoning by other opiates and related narcotics (96509), accidental poisoning—heroin (E8500), accidental poisoning by methadone (E8501), or accidental poisoning—opiates not elsewhere classified (E8502).25,26
Statistical analysis
For each outcome, we estimated the following enrollee/month-level regression: Yit = β0 + β1STATEi + β2POSTit + β3STATEi × POSTit + β4TRENDt + β5STATEi × TRENDt + εit. Sub-scripts i and t represents each enrollee and month. STATEi = 1 if individual i is from Oregon, and 0 if the individual is from Colorado. POSTt = 1 if the observation occurs after July 2012 and 0 otherwise. We censored observations from April to June 2012 (3 months) to account for the staggered rollout for long-acting (April 2012) and short-acting (June 2012) opioids. TRENDt represents a linear trend for each month, beginning in 2011 and running through 2013 (0, 1, 2, …, 35, 36). β3 is the difference-in-differences estimate and is a measure of the change in Oregon following the high dosage policy relative to the difference in Colorado at the same time. We included the interaction of STATE and TREND because trends between states were not parallel. We used a linear probability model for estimation to aid in interpretation of the interaction term27 and cluster standard errors on each enrollee. Finally, we used propensity scores to weight the Colorado population so that they were similar in observed covariates to the Oregon population.28 Covariates for the propensity score model included age, gender, component conditions of a modified version of the Gagne comorbidity score, and the presence of several mental health diagnoses as described in Online Supplement eTable 3.29 Alcohol abuse was omitted from the Gagne score because of substance use disorder data suppression in Colorado data.30
To analyze high dosage opioid use at any point before and after the policy, we used the same approach except that each individual had only 2 observations (pre and post policy). For our analysis of the effect of the policy on a cohort of pre-implementation high dosage opioid users, we restricted the sample to post-policy observations of the high dosage opioid user cohort and then performed 2 multivariate logistic regressions. First, we regressed any post-policy opioid use on STATE to determine if the policy was associated with discontinuation of opioid therapy. Next, we conducted another logistic regression to determine if those who remained on an opioid reduced their dose below 120 mg per day MED. Both regressions were adjusted using the same propensity score weighting technique. Analyses were performed using Stata (StataCorp, College Station, TX). This study was approved by the Oregon Health & Science University Institutional Review Board (IRB00011118).
Results
Table 1 summarizes patient characteristics for the primary analysis, the unadjusted standardized differences between states, and the propensity score–weighted standardized differences. The sample from Oregon was substantially smaller and more severely ill than Colorado. After propensity score weighting, the Oregon and Colorado populations were similar in observed covariates, with standardized differences exhibiting an absolute value of 0.03 or lower.
Table 1.
Summary of demographic and comorbidity characteristics in full and propensity-adjusted population.
| Characteristic | Oregon (N = 24,973) | Colorado (N = 291,411) | Unadjusted standardized difference | Weighted Colorado | Propensity-weighted standardized difference |
|---|---|---|---|---|---|
| Mean age (SD) | 33.5 (15.0) | 31.9 (17.5) | 0.10 | 33.3 (17.5) | 0.02 |
| Female gender | 69.8% | 67.4% | 0.05 | 70.0% | 0.00 |
| Gagne comorbidity components | |||||
| Metastatic cancer | 2.5% | 0.8% | 0.13 | 2.6% | −0.01 |
| Congestive heart failure | 5.2% | 3.2% | 0.10 | 5.3% | −0.01 |
| Dementia | 0.3% | 0.5% | −0.03 | 0.3% | 0.00 |
| Renal failure | 3.2% | 2.0% | 0.08 | 3.2% | 0.00 |
| Weight loss | 2.1% | 1.0% | 0.09 | 2.2% | 0.00 |
| Hemiplegia | 2.4% | 1.0% | 0.11 | 2.5% | −0.01 |
| Any tumor | 6.0% | 2.7% | 0.16 | 6.7% | −0.03 |
| Cardiac arrhythmias | 8.1% | 5.6% | 0.10 | 8.7% | −0.02 |
| Pulmonary disease | 24.0% | 15.1% | 0.22 | 25.2% | −0.03 |
| Coagulopathy | 3.8% | 2.0% | 0.10 | 4.0% | −0.01 |
| Complicated diabetes | 4.1% | 2.2% | 0.10 | 4.0% | 0.00 |
| Anemia | 12.0% | 7.7% | 0.15 | 12.1% | 0.00 |
| Fluid and electrolyte disorders | 15.0% | 12.2% | 0.08 | 15.6% | −0.02 |
| Liver disease | 7.4% | 3.3% | 0.18 | 7.4% | 0.00 |
| Peripheral vascular disease | 2.6% | 1.7% | 0.07 | 2.5% | 0.00 |
| Psychosis | 20.1% | 7.5% | 0.37 | 20.6% | −0.01 |
| Pulmonary circulation disorders | 1.2% | 1.1% | 0.01 | 1.2% | 0.00 |
| HIV/AIDS | 0.7% | 0.2% | 0.06 | 0.7% | −0.01 |
| Hypertension | 21.4% | 13.1% | 0.22 | 21.9% | −0.01 |
| Mental health diagnoses | |||||
| Attention-deficit/hyperactivity disorders | 6.2% | 2.0% | 0.21 | 6.3% | 0.00 |
| Adjustment disorder | 6.7% | 0.6% | 0.33 | 7.2% | −0.02 |
| Anxiety disorder | 26.7% | 9.4% | 0.46 | 28.0% | −0.03 |
| Bipolar disorder | 5.8% | 1.6% | 0.23 | 5.8% | 0.00 |
| Depression | 31.5% | 10.7% | 0.53 | 32.8% | −0.03 |
| PTSD | 10.1% | 1.3% | 0.39 | 10.2% | 0.00 |
| Schizophrenia | 2.6% | 1.0% | 0.11 | 2.5% | 0.00 |
Note. PTSD = posttraumatic stress disorder.
The difference-in-differences analyses of opioid and other medication utilization are summarized in Table 2. Although total opioid use declined significantly in Oregon, it also did so in Colorado, resulting in a nonsignificant net change in monthly opioid use. As shown in Figure 1, the estimated monthly probability of an opioid prescription over 120 mg per day MED was reduced by 1.7 percentage points (95% confidence interval [CI]: −2.0% to −1.4%) following policy implementation, a 53% reduction in baseline high dosage opioid use. There was a significant increase in monthly opioid prescriptions less than 61 mg per day MED of 1.0 percentage points (95% CI: 0.4% to 1.7%). We also observed a significant increase in medications for neuropathic pain (1.2%; 95% CI: 0.7% to 1.8%) and a decline in benzodiazepines (−0.7%; 95% CI: −1.2% to −0.2%). Online Supplemental 1 depicts these trends.
Table 2.
Difference-in-differences analysis of opioid and other medication prescriptions in Oregon relative to Colorado.
| Prescription | State | Pre | Post | Difference | Difference-in-differences (95% confidence interval) | Relative change (95% confidence interval) |
|---|---|---|---|---|---|---|
| Opioids | ||||||
| Total | Oregon | 0.2481*** | 0.2427*** | −0.0054* | 0.0021 | 0.8% |
| (0.0026) | (0.0025) | (0.0031) | (−0.0047 to 0.0089) | (−1.9% to 3.6%) | ||
| Colorado | 0.1883*** | 0.1809*** | −0.0074*** | |||
| (0.0014) | (0.0014) | (0.0016) | ||||
| >120 MED | Oregon | 0.0321 *** | 0.0143*** | −0.0179*** | −0.0174*** | −53.0% |
| (0.0014) | (0.0009) | (0.0014) | (−0.0204 to −0.0143) | (−63.6 to −44.5) | ||
| Colorado | 0.0247*** | 0.0242*** | −0.0005 | |||
| (0.0007) | (0.0006) | (0.0008) | ||||
| 61–120 MED | Oregon | 0.0537*** | 0.0548*** | 0.0012 | 0.0011 | 2.0% |
| (0.0016) | (0.0015) | (0.0019) | (−0.0031 to 0.0052) | (−5.8% to 9.7%) | ||
| Colorado | 0.0420*** | 0.0421 *** | 0.0001 | |||
| (0.0007) | (0.0007) | (0.0009) | ||||
| <61 MED | Oregon | 0.2042*** | 0.2072*** | 0.0030 | 0.0101*** | 4.9% |
| (0.0025) | (0.0024) | (0.0030) | (0.0035 to 0.0168) | (1.7% to 8.2%) | ||
| Colorado | 0.1604*** | 0.1533*** | −0.0071*** | |||
| (0.0012) | (0.0012) | (0.0015) | ||||
| Other drug classes | ||||||
| Arthritis | Oregon | 0.0806*** | 0.0826*** | 0.0020 | 0.0027 | 3.3% |
| (0.0017) | (0.0017) | (0.0020) | (−0.0016 to 0.007) | (−2.0% to 8.7%) | ||
| Colorado | 0.0563*** | 0.0557*** | −0.0007 | |||
| (0.0008) | (0.0007) | (0.0010) | ||||
| Neuropathic pain | Oregon | 0.1374*** | 0.1461*** | 0.0087*** | 0.0123*** | 9.0% |
| (0.0025) | (0.0026) | (0.0025) | (0.0066 to 0.0181) | (4.8% to 13.2%) | ||
| Colorado | 0.1103*** | 0.1066*** | −0.0037** | |||
| (0.0014) | (0.0014) | (0.0015) | ||||
| Benzodiazepines | Oregon | 0.0703*** | 0.0700*** | −0.0003 | −0.007*** | −10.0% |
| (0.0018) | (0.0018) | (0.0019) | (−0.0116 to −0.0024) | (−16.5% to −3.4%) | ||
| Colorado | 0.0737*** | 0.0804*** | 0.0067*** | |||
| (0.0012) | (0.0012) | (0.0015) |
Note. MED = morphine equivalent daily dose (mg). Estimates indicate monthly predicted probabilities with standard errors in parentheses unless indicated otherwise. Relative change is relative percentage change in Oregon following the high dosage policy.
P < .0001;
P < .001;
P < .05.
Figure 1.
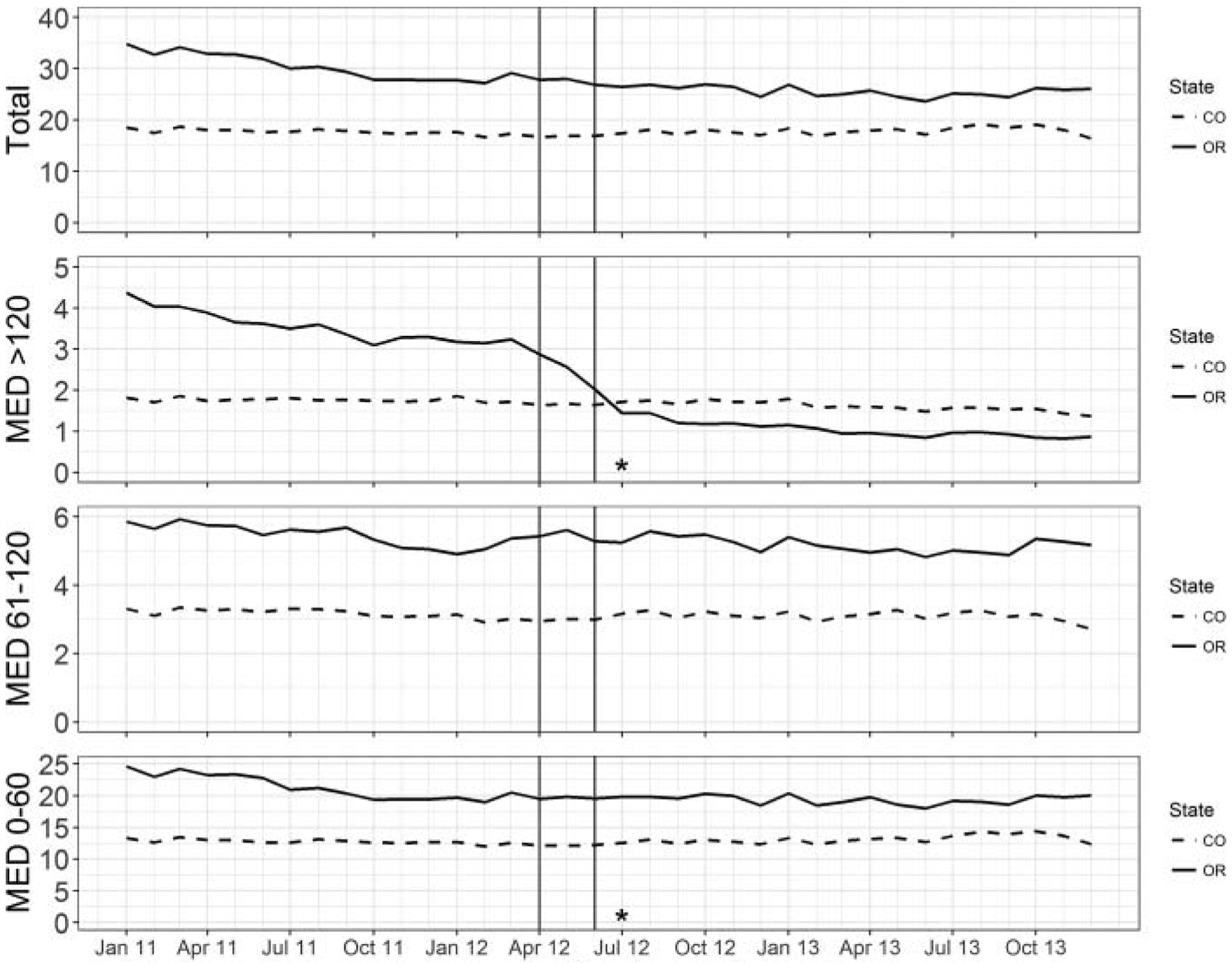
Opioid prescriptions filled per 100 enrolled individuals (total, >120 MED, 61–120 MED, and <61 MED) from January 2011 to December 2013 in Oregon and Colorado. Solid line indicates Oregon, and dashed line indicates Colorado; vertical lines indicate policy implementation period April 2012 to June 2012. MED = morphine equivalent dose per day. *P < 0.05.
As shown in Table 3 (Online Supplemental eFigure 2 and 3), there were no significant changes related to any high-risk opioid use indicators, with the exception of multiple pharmacy use, which declined by 0.1 percentage points (95% CI: −0.02% to −0.001%). There were no changes in opioid-related ED visits or hospitalizations (Table 4 and Online Supplemental eFigure4).
Table 3.
Difference-in-differences analysis of high-risk opioid use indicators in Oregon relative to Colorado.
| Opioid use | State | Pre | Post | Difference | Difference-in-differences (95% confidence interval) | Relative change (95% confidence interval) |
|---|---|---|---|---|---|---|
| Opioid-opioid overlap | Oregon | 0.0337*** | 0.0324*** | −0.0012 | −0.008 | −2.4% |
| (0.0011) | (0.0010) | (0.0013) | (−0.0038 to 0.0022) | (−11.3% to 6.5%) | ||
| Colorado | 0.0341*** | 0.0337*** | −0.0004 | |||
| (0.0005) | (0.0006) | (0.0007) | ||||
| Opioid-benzodiazepine overlap | Oregon | 0.0246*** | 0.0252*** | 0.0007 | 0.0008 | 3.3% |
| (0.0010) | (0.0010) | (0.0012) | (−0.002 to 0.0036) | (−8.1% to 14.6%) | ||
| Colorado | 0.0237*** | 0.0235*** | −0.0001 | |||
| (0.0006) | (0.0006) | (0.0009) | ||||
| Opioid-nonbenzodiazepine sedative | Oregon | 0.0108*** | 0.0091*** | −0.0017** | −0.0013 | −9.3% |
| (0.0006) | (0.0006) | (0.0008) | (−0.0031 to 0.0005) | (−28.7% to 4.6%) | ||
| Colorado | 0.0130*** | 0.0126*** | −0.0004 | |||
| (0.0004) | (0.0004) | (0.0005) | ||||
| Long-acting opioid use after acute pain diagnosis | Oregon | 0.0355*** | 0.0338*** | −0.0016 | −0.0007 | −2.0% |
| (0.0010) | (0.0009) | (0.0015) | (−0.0041 to 0.0027) | (−11.5% to 7.6%) | ||
| Colorado | 0.0281 *** | 0.0271*** | −0.0009 | |||
| (0.0006) | (0.0006) | (0.0009) | ||||
| Multiple pharmacy use | Oregon | 0.0016*** | 0.0012*** | −0.0005 | −0.001** | −62.5% |
| (0.0002) | (0.0001) | (0.0003) | (−0.0019 to −0.0000) | (−118.8% to 0%) | ||
| Colorado | 0.0050*** | 0.0055*** | 0.0005 | |||
| (0.0003) | (0.0003) | (0.0004) |
Note. Estimates indicate monthly predicted probabilities with standard errors in parentheses unless indicated otherwise. Relative change is relative percentage change in Oregon following the high dosage policy.
P < .001;
P < .05.
Table 4.
Difference-in-differences analysis of opioid-related emergency department (ED) visits or hospitalizations.
| ED visit or hospitalization | State | Pre | Post | Difference | Difference-in-differences (95% confidence interval) | Relative change (95% confidence interval) |
|---|---|---|---|---|---|---|
| ED visit | Oregon | 0.0002*** | 0.0001*** | −0.0001 | −0.0001 | −50% |
| (0.0001) | (0.0000) | (0.0001) | (−0.0003 to 0.0001) | (−150% to 50%) | ||
| Colorado | 0.0001*** | 0.0001*** | −0.0000 | |||
| (0.0000) | (0.0000) | (0.0000) | ||||
| Hospitalization | Oregon | 0.0001** | 0.0001*** | 0.0000 | 0.0000 | 0% |
| (0.0000) | (0.0000) | (0.0001) | (−0.0001 to 0.0001) | (−100% to 100%) | ||
| Colorado | 0.0001*** | 0.0001*** | 0.0000 | |||
| (0.0000) | (0.0000) | (0.0000) | ||||
| ED visit or hospitalization | Oregon | 0.0002*** | 0.0001*** | −0.0001 | −0.0001 | −50% |
| (0.0001) | (0.0000) | (0.0001) | (−0.0003 to 0.0001) | (−150% to 50%) | ||
| Colorado | 0.0001*** | 0.0001*** | 0.0000 | |||
| (0.0000) | (0.0000) | (0.0000) |
Note. Estimates indicate monthly predicted probabilities with standard errors in parentheses unless indicated otherwise. Relative change is relative percentage change in Oregon following the high dosage policy.
P < .0001;
P < .001;
P < .05.
Table 5 summarizes changes in any high dosage opioid use at any point and among those individuals who filled a high dosage opioid prescription prior to the policy. Following implementation of the high dosage policy, the estimated probability of filling a high dosage opioid prescription was reduced by 4.3 percentage points (66% decrease from the pre-policy likelihood; 95% CI: −4.8% to −3.8%). Among those with a high dosage opioid prescription in Oregon prior to the policy, 90.4% had an opioid fill after the policy, which was significantly higher than the proportion in Colorado (79.1%). However, among those who continued on an opioid after the policy, the probability of it being high dosage declined by 20.3 percentage points (95% CI: −25.3% to −15.3%) compared with Colorado, a 35% relative decline.
Table 5.
Difference-in-differences analysis of any high dosage opioid prescription and logistic regression of the disposition of the high dosage user cohort.
| State | Pre | Post | Difference | Difference-in-differences (95% confidence interval) | Relative change (95% confidence interval) |
|---|---|---|---|---|---|
| Any high dosage opioid fill (>120 MED) | |||||
| Oregon | 0.0648*** (0.0017) |
0.0139*** (0.0009) |
−0.0509*** (0.0017) |
−0.0429*** (−0.048 to −0.0379) | −66.2% (−74.1% to −58.5%) |
| Colorado | 0.0920*** (0.0015) |
0.0840*** (0.0015) |
−0.0080*** (0.0019) |
||
| Disposition of high dosage opioid user prior to the policy | |||||
| Probability of any opioid use in post | Difference (95% confidence interval) | Relative difference (95% confidence interval) | |||
| Oregon | 0.9041*** (0.0136) |
0.1131*** (0.0829 to 0.143) |
14.3% (10.5% to 18.1%) | ||
| Colorado | 0.7910*** (0.0073) |
||||
| Probability of high dosage opioid use in post | Difference (95% confidence interval) | Relative Difference (55% Confidence Interval) | |||
| Oregon | 0.3741*** (0.0231) |
−0.2032*** (−0.253 to −0.153) |
−35.2% (−43.8% to −26.5%) | ||
| Colorado | 0.5774*** (0.0108) |
||||
Note. MED = morphine equivalent daily dose (mg). Estimates indicate predicted probabilities with standard errors in parentheses unless indicated otherwise. Relative change is relative percentage change in Oregon following the high dosage policy. Relative difference is relative percentage difference between Oregon and Colorado.
P < .05.
Discussion
Our study shows that Oregon’s prior authorization policy resulted in a significant decline in fills for high dosage opioids. The policy was associated with a significant increase in the likelihood of filling opioids at a lower dosage (<61 mg per day MED) to an extent similar as the decline in high dosage opioid filling (>120 mg MED), suggesting tapering to lower dose. These changes in opioid utilization translated into a 66% relative reduction in the likelihood of an individual filling a high dose opioid after the policy. Although high dosage opioid users in Oregon were more likely to continue on an opioid compared with those in Colorado, they were more likely to do so at a lower dosage. Following the policy, there was also a reduction in the rate of opioids fills from multiple pharmacies. It is conceivable that individuals who are prescribed high dosage opioids may also engage in other behaviors perceived to be high risk. A study by Yang et al. found that Medicaid patients categorized as pharmacy shoppers with overlapping opioids were more likely to be prescribed a dosage above 100 mg per day MED than control subjects (non-pharmacy shoppers and no overlapping opioids).31 It is plausible that policy-related declines in high dosage opioid use may have indirectly reduced multiple pharmacy use metrics. We found no evidence of a decrease in health outcomes related to opioid poisoning, although the number of opioid-related outcomes was low.
We observed a significant increase in the use of potentially substitutable medications for neuropathic pain. Interestingly, we also observed a net decline in benzodiazepine use following policy implementation. Although the most likely explanation is an unexplained significant increase in benzodiazepine use in Colorado, it is important to highlight that benzodiazepine utilization does not appear to increase in Oregon as a consequence of the policy.
This study adds to a sparse literature evaluating the effects of payer mechanisms aimed at improving medication safety.32,33 Although various reports describe organizational efforts and guidelines to reduce prescribing high dosage of opioids, there are few studies examining prior authorization policies. The only other study to document the effect of opioid dosing limits originated from the Massachusetts Medicaid program,19 where a high dosage opioid prior authorization policy was applied to long-acting agents and the daily dosage criteria were much higher (240 mg of oxycodone, 200 μg of fentanyl, 360 mg of morphine, and 120 mg of methadone). Investigators found that the policy was associated with average dosage reductions for methadone and extended-release morphine. However, they also observed increases for fentanyl and extended-release oxycodone that may have been due to another policy that required a trial of methadone or morphine prior to use of these agents. The effect of the policy on non–pharmacy utilization outcomes was not evaluated.
One of the most comprehensive analyses of initiatives to reduce high dosage opioid use examined the state of Washington’s Interagency Guideline on Opioid Dosing for Chronic Non-Cancer Pain, released in 2007.15 A key feature was the recommendation that patients requiring opioid dosage in excess of 120 mg per day MED consult with a pain medicine specialist. Several studies derived from Medicaid and Workers’ Compensation populations suggest that guideline-related educational efforts were associated with reductions in high dosage opioid use, chronicity of use, and potentially opioid-related poisoning.16,25,34,35 However, because these studies did not include a “control group,” it is unclear if observed trends were causally related to guideline dissemination or secular trends in prescribing and overdose. Nationwide, the rate of prescription opioid-related deaths has slowed somewhat over the last 5 years.36,37
This study has several limitations that merit consideration. A fundamental limitation of all studies that use administrative claims data is that utilization that is not reimbursed by a third-party payer is not observed. It is plausible that patients who are denied reimbursement simply pay out-of-pocket. The advent of, and increasing access to, controlled substances fill data from state prescription drug monitoring programs could potentially allow investigators to explore this phenomena in more depth. A recent analysis using state prescription drug monitoring program data in North Carolina demonstrated increasing rates of out-of-pocket payments among Medicaid recipients who were enrolled in the state’s lock-in program.38 Next, although we compared 2 state Medicaid programs, there were substantial differences in our populations in both size and severity of illness. These differences can largely be attributed to the use of a fee-for-service population in Oregon. In Oregon, each specific managed care organization develops their own pharmacy benefit policies and the high dosage policy was exclusive to the fee-for-service population. In contrast, Colorado uses a uniform pharmacy benefit plan across most of its Medicaid program (both managed care and fee-for-service). To mitigate this imbalance, we employed weighted propensity scores to adjust our difference-in-differences analysis. Despite this adjustment, we recognize that residual differences likely remain between our study populations. Third, we used an overdose outcome that was predicated on generation of a Medicaid claim. As a result, we are likely missing data for fatal overdoses where health care claims in the ED or hospital were not submitted. Also, overdose events were relatively rare, and our analysis was likely underpowered. This study was confined to a Medicaid population, and findings may not translate to other populations. However, studies in other drug classes consistently demonstrate prior authorization policies to be potent drivers of pharmacy utilization in a diversity of settings.39 Finally, the use of administrative data preclude measurement of patient-reported outcomes such as pain, quality of life, or functional status. A small study of a high dosage policy implemented in a small number of internal medicine clinics in Oregon using electronic health record data suggests no gross deterioration of pain or quality-of-life scores, but larger and more robust studies of similar policies are required to confirm this in the future.40
The recent release of the Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain was a watershed moment as the United States mounts initiatives to combat the prescription opioid overdose epidemic.41 In addition to addressing issues related to decisions to begin opioid therapy and product selection (short-versus long-acting), the guideline also recommends clinicians use caution when prescribing over 50 mg per day MED and avoid prescribing above 90 mg per day MED or carefully justify a decision to titrate to this dosage. Although a variety of organizations have provided dosage thresholds to reduce the risks of misuse, abuse, and overdose,42 the CDC guideline is the first national guideline that uses the most recent scientific evidence to inform dosage recommendations for patients on long-term opioid therapy for chronic pain. These recommendations concern some who believe that the recommendations may cause unintended harm to patients with chronic pain who may now see increased barriers to otherwise appropriate and responsible care.43 Specifically, payers and other organizations may use CDC thresholds as a basis for coverage decisions using policies similar to Oregon’s. One critique of Oregon’s high dosage prior authorization is that it was not accompanied by other resources to assist patients or providers who may have needed to reduce or discontinue opioids. This is in contrast to a recent effort in Oregon’s Medicaid program to reduce opioid use for individuals with back or spine conditions that concomitantly expands coverage for alternative treatment options such as acupuncture, chiropractic services, and physical therapy.44
In summary, Oregon’s high dosage opioid prior authorization policy was associated with utilization changes consistent with opioid tapering and potential substitution with non–opioid-related medications. Although the policy was also associated with reduced multiple pharmacy use, we found no evidence of reduced opioid-related adverse outcomes. Current trends suggest that heroin and illicit fentanyl are the primary drivers of opioid-related overdoses in many areas of the country.45 Consequently, efforts to curtail prescription opioids may have limited impact on opioid-related overdose outcomes.
Supplementary Material
Funding
This work is supported through a cooperative agreement with the Centers for Disease Control and Prevention in Atlanta, Georgia (1U011CE002500). The Centers for Disease Control and Prevention had no role in the design and conduct of the study; collection, management, and analysis of data; approval of the manuscript; and decision to submit the manuscript for publication. The Centers for Disease Control and Prevention reviewed and provided input in the interpretation of the data; and preparation and revisions of the manuscript. The conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The authors declare that they have no conflicts of interest.
Supplemental data for this article can be accessed on the publisher’s website.
Deaths involving prescription opioids are identified using International Classification of Diseases, Tenth Revision underlying cause-of-death codes X40–X44, X60–X64, X85, and Y10–Y14 with a multiple cause code of T40.0, T40.2, T40.3, or T40.4.
References
- [1].Centers for Disease Control and Prevention. Wide-ranging Online Data for Epidemiologic Research (WONDER). Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- [2].Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 2015;175:608–615. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- [3].Paulozzi LJ, Zhang K, Jones CM, Mack KA. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J Am Board Fam Med. 2014;27:329–338. doi: 10.3122/jabfm.2014.03.130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104: e32–e42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bohnert AS, Logan JE, Ganoczy D, Dowell D. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care. 2016;54:435–441. doi: 10.1097/MLR.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- [7].Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17:85–98. [DOI] [PubMed] [Google Scholar]
- [8].Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- [10].Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- [11].Liang Y, Turner BJ. Assessing risk for drug overdose in a national cohort: role for both daily and total opioid dose? J Pain. 2015;16:318–325. doi: 10.1016/j.jpain.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paulozzi LJ, Kilbourne EM, Shah NG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- [13].Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15:1911–1929. doi: 10.1111/pme.12480. [DOI] [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention. Common elements in guidelines for prescribing opioids for chronic pain. http://www.cdc.gov/drugoverdose/pdf/common_elements_in_guidelines_for_prescribing_opioids-a.pdf. Accessed July 11, 2016.
- [15].Franklin G, Sabel J, Jones CM, et al. A comprehensive approach to address the prescription opioid epidemic in Washington State: milestones and lessons learned. Am J Public Health. 2015;105:463–469. doi: 10.2105/AJPH.2014.302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sullivan MD, Bauer AM, Fulton-Kehoe D, et al. Trends in opioid dosing among Washington state Medicaid patients before and after opioid dosing guideline implementation. J Pain. 2016;May;17 (5):561–8. doi: 10.1016/j.jpain.2015.12.018. [DOI] [PubMed] [Google Scholar]
- [17].Keast SL, Farmer K, Smith M, Nesser N, Harrison D. Prior authorization policies in Medicaid programs: the importance of study design and analysis on findings and outcomes from research. Res Soc Admin Pharm. 2016;12:154–163. doi: 10.1016/j.sapharm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- [18].Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46:573–580. [DOI] [PubMed] [Google Scholar]
- [19].Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20:447–454. doi: 10.18553/jmcp.2014.20.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oregon Medicaid Fee-For-Service Prior Authorization Criteria. Oregon Health Authority. http://www.oregon.gov/oha/healthplan/tools/Oregon%20Medicaid%20PA%20Criteria,%20June%202012.pdf. Published 2012. Accessed February 27, 2017. [Google Scholar]
- [21].Hartung DM, Zerzan J, Yamashita T, et al. Characteristics and trends of low-dose quetiapine use in two western state Medicaid programs. Pharmacoepidemiol Drug Saf. 2014;23:87–94. doi: 10.1002/pds.3538. [DOI] [PubMed] [Google Scholar]
- [22].National Center for Injury Prevention and Control. CDC Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics With Oral Morphine Milligram Equivalent Conversion Factors, 2016 version. http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Published 2016. Accessed March 23, 2017.
- [23].Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19:648–665. [PubMed] [Google Scholar]
- [24].Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50:494–500. doi: 10.1097/MLR.0b013e31824ebd81. [DOI] [PubMed] [Google Scholar]
- [25].Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington State Medicaid: trends, dosing, and guidelines. Med Care. 2015;53:679–685. doi: 10.1097/MLR.0000000000000384. [DOI] [PubMed] [Google Scholar]
- [26].Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern. Med 2015;175:978–987. doi: 10.1001/jamainternmed.2015.0914. [DOI] [PubMed] [Google Scholar]
- [27].Ai C, Norton EC. Standard errors for the retransformation problem with heteroscedasticity. J Health Econ. 2000;19:697–718. doi: 10.1016/S0167-6296(00)00046-1. [DOI] [PubMed] [Google Scholar]
- [28].Stuart EA, Huskamp HA, Duckworth K, et al. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv Outcomes Res Methodol. 2014;14:166–182. doi: 10.1007/s10742-014-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frakt AB, Bagley N. Protection or Harm? Suppressing substanceuse data. N Engl J Med. 2015;372:1879–1881. doi: 10.1056/NEJMp1501362. [DOI] [PubMed] [Google Scholar]
- [31].Yang Z, Wilsey B, Bohm M, et al. Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long-term opioid users in Medicaid. J Pain. 2015;16:445–453. doi: 10.1016/j.jpain.2015.01.475. [DOI] [PubMed] [Google Scholar]
- [32].Ross JS, Jackevicius C, Krumholz HM, et al. State Medicaid programs did not make use of prior authorization to promote safer prescribing after rosiglitazone warning. Health Aff. 2012;31:188–198. doi: 10.1377/hlthaff.2011.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reinke T Prior authorization for safety, not just for economy. Manag Care. 2012;21:33–35. [PubMed] [Google Scholar]
- [34].Garg RK, Fulton-Kehoe D, Turner JA, et al. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14:1620–1628. doi: 10.1016/j.jpain.2013.08.001. [DOI] [PubMed] [Google Scholar]
- [35].Fulton-Kehoe D, Garg RK, Turner JA, et al. Opioid poisonings and opioid adverse effects in workers in Washington State. Am J Ind Med. 2013;56:1452–1462. doi: 10.1002/ajim.22266. [DOI] [PubMed] [Google Scholar]
- [36].Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- [37].Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- [38].Roberts AW, Farley JF, Holmes GM, et al. Controlled substance lock-in programs: examining an unintended consequence of a prescription drug abuse policy. Health Aff. 2016;35:1884–1892. doi: 10.1377/hlthaff.2016.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Green CJ, Maclure M, Fortin PM, et al. Pharmaceutical policies: effects of restrictions on reimbursement. Cochrane Database Syst Rev. 2010;(8):CD008654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weimer MB, Hartung DM, Ahmed S, Nicolaidis C. A chronic opioid therapy dose reduction policy in primary care. Subst Abus. 2016;37:141–147. doi: 10.1080/08897077.2015.1129526. [DOI] [PubMed] [Google Scholar]
- [41].Dowell D, Haegerich TM, Chou R, et al. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ziegler SJ. The Proliferation of dosage thresholds in opioid prescribing policies and their potential to increase pain and opioid-related mortality. Pain Med. 2015;16:1851–1856. doi: 10.1111/pme.12815. [DOI] [PubMed] [Google Scholar]
- [43].Tavernise S. C.D.C. painkiller guidelines aim to reduce addiction risk. New York Times. March 15, 2016. https://www.nytimes.com/2016/03/16/health/cdc-opioid-guidelines.html [Google Scholar]
- [44].Oregon Health Authority. Back conditions technical changes. http://www.oregon.gov/oha/herc/FactSheet/Back-line-and-guideline-tech-summary.pdf. Published 2016. Accessed March 22, 2017.
- [45].Kertesz SG. Turning the tide or riptide? The changing opioid epidemic. Subst Abus. 2017;38:3–8. doi: 10.1080/08897077.2016.1261070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


