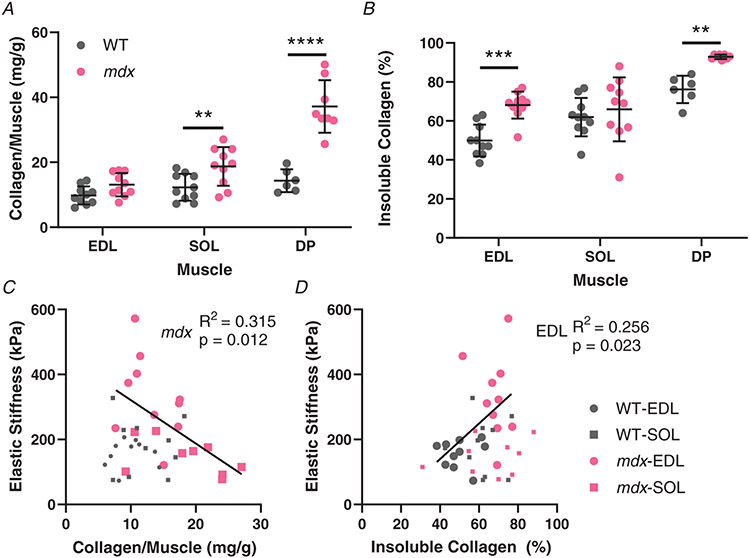
Figure 4. Collagen content and solubility, determined through a hydroxyproline assay, and its relation to passive mechanics.
A, the amount of collagen per muscle in wildtype and mdx muscles. The soleus and especially diaphragm muscle collagen content of mdx mice was significantly increased compared with the wildtype. Diaphragm muscles were not collected from all mice (n = 6 wildtype; n = 8 mdx). B, the percentage of collagen that was pepsin insoluble as an indication of crosslinked collagen per muscle in wildtype and mdx muscles. Contrasting with total collagen, EDL muscle had significantly more crosslinked collagen compared while there was no difference in soleus. The diaphragm muscle was the most crosslinked with a highly significant increase in crosslinking in mdx diaphragms. C, the relationship between the amount of collagen per muscle and muscle elastic stiffness. There is a significant negative relationship for the mdx muscles, demonstrating that increased fibrotic material does produce stiffness in fibrosis. D, the relationship between elastic stiffness and collagen insolubility per muscle. The percentage of soluble collagen and elastic stiffness had a significant positive correlation, indicating a link between stiffness and crosslinking in the EDL. **P < 0.01, ***P < 0.001 and ****P < 0.0001.