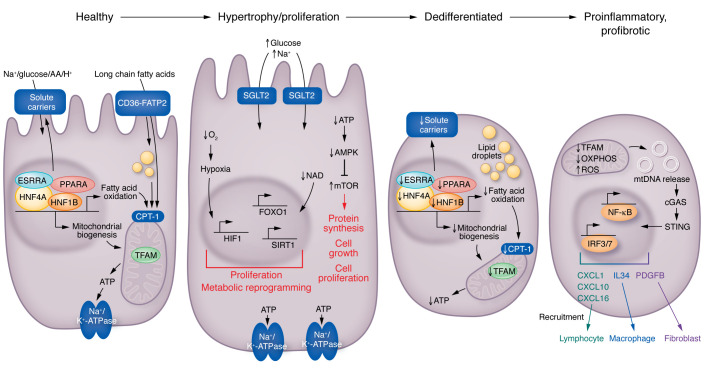
Figure 3. Changes in kidney proximal tubule cells in diabetes.
Healthy PT cells utilize fatty acids and generate ATP via mitochondrial oxidative phosphorylation to support the Na+/K+ ATP-ase, which then creates a sodium gradient for sodium-mediated glucose, amino acid, or proton reabsorption. Increased tubule glucose presents an increased load for the basal Na+/K+ ATPase and the sodium-mediated glucose cotransporter; more oxygen and ATP are needed to meet this higher metabolic need. The higher AMP/ATP ratio is sensed by AMPK. This excess workload will result in reduced oxygen concentration (relative hypoxia), which is sensed by hypoxia-inducible factor (HIF). HIF activation will induce metabolic reprogramming, and, together with mTOR, will induce tubule cell proliferation and hypertrophy, proliferation, and kidney growth. Later stages of DKD are characterized by dedifferentiation of PT cells. Gene programs associated with mitochondrial biogenesis, fatty acid oxidation, mitochondrial function, and low ATP levels lead to the loss of solute carriers and cellular dedifferentiation. Finally, at later stages of DKD, severe mitochondrial damage will lead to the release of mitochondrial DNA (mtDNA) into the cytosol. Cystosolic mtDNA is then sensed by nucleotide sensing pathways, such as cGAS and stimulator of interferon genes (STING), inducing the activation of IRF3/7 and NF-κB pathways and resulting in transcription of cytokines, growth factors, and downstream immune cell recruitment and fibroblast activation. CPT-1, carnitine palmitoyltransferase 1A; ESRRA, estrogen-related receptor alpha; FATP2, fatty acid transport protein 2; HNF1B, hepatocyte nuclear factor 1B; IRF3/7, inferferon regulatory factor 3/7.