Abstract

The quest for clean energy conversion has become one of the most important efforts for tackling the greenhouse effect for a sustainable environment. This involves energy-scavenging processes like photovoltaics and catalysis, which have been manifested using the solar spectrum. For high-efficiency and durable conversion processes, the search for the low-cost, stable, and environment-friendly functional materials is elusive. In the field of solar cells and catalysis, double perovskite oxides (DPOs) have emerged as potential candidates in recent years. Through compositional tuning and band gap engineering, a plethora of materials are being developed for pertinent applications in this field of energy. Oxide perovskites possess the advantage of a high carrier lifetime compared to that with halide perovskites, which can be beneficial for energy applications. In this perspective, we have presented theoretical investigations focusing on the different types of double perovskite oxides based on the composition space in a systematic manner. Corresponding electronic and optical properties are discussed along with a future outlook on the novel routes to find efficient members in this family.
Keywords: double perovskite oxides, cation occupancy, alkaline-earth, transition metal, rare earth, mixed cation/anion
Introduction
An increase in energy demand globally has inspired the research community to determine better materials for sustainable and clean energy along with reducing concerns about environmental pollution from fossil energy. In this regard, perovskite materials have been established as a family of materials for energy conversion and storage.1−4 Any mineral with an ABX3 structural framework is known as perovskite, with CaTiO3 being the first to be discovered. Here, A is a cation, B is usually a metallic cation, and X is oxygen or halogen. Extensive studies on improving these perovskite materials have extrapolated into developing double perovskites with A2BB′X6 stoichiometry.5 The oxide family of double persovskites with A2BB′O6 stoichiometry6−8 have much advanced dominance in the field of energy harvesting, which includes solar cells, light-emitting diodes, and photocatalysis.9,10 In the field of solar cells, the charge-carrier recombination can be envisaged, whereas in photocatalysis, the photoexcited electrons are the prominent governing factors. In this regard, double perovskite oxides (DPOs) have emerged as a promising materials family for such energy applications, while a profound understanding is required from the electronic structure perspective in order to explore their ability to be used as highly efficient photovoltaic and photocatalytic materials. We have witnessed a series of experimental and theoretical investigations on DPOs where the structural, electronic, and optical properties have been manifested in the last couple of decades. In this perspective, we have taken a constructive theoretical viewpoint regarding the electronic and optical properties of DPOs, while exploring the compositional space and stoichiometry of these materials classified as alkaline earth–transition metal–rare earth–mixed anion/cation-based DPOs. A schematic representation of the classification of DPOs in our perspective is shown in Figure 1.
Figure 1.
Representation of the classification of double perovskite oxides with the material class and pictorial representation of an example structure in each case.
In alkaline earth double perovskite oxides (AA′BB′O6), the A- and A′-sites with a valence of +1/+2 belong to the first or second group of the periodic table (K, Ba, Sr, etc.), whereas B- and B′-sites mostly have variable oxidation states (+3, +4, +5, +6) combined stoichiometrically.11,12 Apart from this, there is a constant ongoing effort to explore more of its electronic and optical properties through substitution of lighter elements, such as Sb, in place of heavier Bi. For exploring transition-metal-based DPOs, rare-earth-based DPOs, and mixed anion/cation DPOs, researchers have used a combinatorial approach having different proportions. Nowadays, due to data-driven research and development in the field of machine learning (ML) and artificial intelligence (AI), it is very much helpful to study the feasibility and predict the properties of material computationally without it being synthesized physically, giving rise to a huge amount of research opportunity in these areas.
Advanced studies in perovskites reveals the new family of complex DPOs.6,7 In general, the 3d and 4d electron configuration and their multivalency features make transition metal elements the best candidates to be occupied in the B-site cation position.13−15 It is also worthy to note that the transition metal oxides exhibit perovskite-like structure and possess extensive physical properties, making the perovskite constituted with these transition elements a better solar-cell material.16−19 Along with other components, the BX6 octahedral tilt further stabilizes the perovskite oxide structure by lowering the crystal symmetry.10 In addition, the cation ordering in such perovskites also plays a pivotal role in obtaining better stability.
Recently, tremendous efforts have been made to address the energy issues through developing new functional materials. Nevertheless, oxide perovskites and their derivatives have been extensively used in many technologies, reflecting their versatile properties.9,10 Due to their compositional and structural flexibility, oxide perovskites show several other attractive properties including superconductivity, ferroelectricity, magnetoresistance, and ionic conductivity. Although halide perovskites20 have shown considerable promise in photovoltaic (PV) applications due to their superior photovoltaic properties such as extremely high optical absorption coefficient, super long carrier diffusion length, and low-temperature solution processability, the practical use of it and its commercialization is still not always possible because of the instabilities associated with moisture, temperature, and other environmental factors. On the other hand, double perovskite oxides and their derivatives have shown greater applications in electrocatalysis, photocatalysis, and photovoltaics attributed to their superior stability. Here, we provide an overall perspective on the structural and compositional flexibility of DPOs and their derivatives. Further, we discuss the status of their applications in catalysis and photovoltaics. Finally, we also deliver a brief insight into potential DPO-based research fields one could consider exploring in the near future.
Recent Progress in Double Perovskite Oxides
Alkaline-Earth-Based Double Perovskite Oxides
There are several types of complex oxide perovskites. One being the pyrochlore mineral family having the general formula (Na,Ca)2Nb2O6(OH). These pyrochlores can be subclassified as complex oxide perovskites with A2B2O6O′ as the evolved formula.21 Unlike usual perovskites, pyrochlores have two types of anions: one that links the A- and B-site (O) and the other that brings the link between the pair of A-sites (O′). The complex pyrochlore structure perovskites can be subcategorized in six ways: (1) A2B2O6, obtained by a defective or vacant O′ oxide state; (2) A2–xB2O7, arisen due to the partial occupancy of the A-site; (3) the neutral structure, caused by the O′-site occupying balancing anions along with the oxide ions; (4) A2B2O7, due to the O′ migrating under constraints giving rise to partial occupancy for a previously unoccupied oxide site; (5) mixing of the A- and B-site metals; (6) AB2O6 β-pyrochlore, obtained with the metal in A-site residing on the O′-site.11
Another new family of complex double oxide perovskites, AA′BB′O6 (A and A′ are univalent A-site cations; B and B′ are univalent B-site cations), have emerged from recent studies on lead-free perovskites.12 This family of perovskites have proven its successful applications in photovoltaic and optoelectronic industries with improved stability and efficiency. In fact, compared to A2BB′O6, these AA′BB′O6 materials are believed to possess lower band gap addition for improved photovoltaic applications. The same work12 reports that with a simple charge balancing variation, 29,515 unique structures of AA′BBiO6 DPOs can be obtained. Few of the structures can be obtained from the Inorganic Crystal Structure Database (ICSD). One such structure with high thermodynamic stability, KBaTeBiO6, is the first one ever to be reported. In the work by Thind et al.,12 among all the predicted compounds, only two compounds, KBaTeBiO6 and SrBaVBiO6, having negative formation enthalpy in their ground state and showing ideal perovskite structure, are lead-free. SrBaVBiO6 has a flat valence band (VB) and conduction band (CB), making it a nonsuitable photovoltaic material. KBaTeBiO6 has negative formation enthalpy of ΔHf = −39 meV/atom, suggesting it as an interesting system along with the feasibility of it to be synthesized. The same work also reports on the synthesis and analysis of KBaTeBiO6 with a P4/nmm space group and a0a0c0 as the octahedral tilt pattern. The theoretical indirect band gap of KBaTeBiO6 is reported to be 1.94 eV, whereas the experimental indirect band gap is found to be 1.88 eV. However, a theoretical direct band gap is found to be 2.83 eV occurring at Gamma-point. The work12 also predicts that the band structure possesses highly dispersed valence and conduction bands, which are most desirable for faster transport of holes and electrons, making it more interesting. The effective mass of holes and electrons in KBaTeBiO6 is calculated to be 0.25 and 0.28me, respectively (where me is the mass of electrons). This low effective masses of holes and electrons contributes toward achieving better carrier transport. Furthermore, analysis of electronic properties in the KBaTeBiO6 complex DPO has been discussed in the same work by Thind et al.12 Presently, our group is working on further analysis of the system theoretically, and our computationally obtained results for total and projected density of states for KBaTeBiO6 is shown in Figure 2. Analysis of the partial density of states suggests that the O−2p and Bi−6s states contribute to the valence band regime with minor contribution from the Te−4d state, whereas the conduction band is dominated by Bi−6p and Te−5s states with the smaller occupancy by the O−2p state. The presence of the Bi−6s antibonding state in the valence band maxima rather than conduction band minima results in its +3 oxidation state. This oxidation state is further supported by 2.73e empty conduction band states of Bi. These aspects of Bi impact the defect tolerance due to the lone-pair chemistry.
Figure 2.

Computational analysis of KBaTeBiO6 showing total and projected density of states.
Another interesting addition to the work12 is the evaluation of another material configuration,
K0.625Ba1.375Te0.875Bi1.125O6. It is fascinating to see that without introducing
any defect, this
new nonstochiometric system exhibits a reduced band gap of 1.18 eV.
The new system possesses the 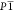 ground-state space group along with an a–b–c– octahedral tilt pattern. The
new system also favors thermodynamic cation nonstoichiometry due to
the calculated formation enthalpy of ΔHf = −148 meV/atom, lower than that of the pristine sample.12 The efficiency of KBaTeBiO6 is calculated
to be 0.057%, which is relatively low; however, it shows much better
stability than many other bismuth-based halide perovskites. Also,
solar cells composed of KBaTeBiO6 nanoparticles exhibit
a performance similar to those based on high-quality thin films of
other inorganic oxide perovskites. Much higher efficiency of the solar
cells based on KBaTeBiO6 can be achieved by controlling
and optimizing the stoichiometry and growing high-quality thin films
using aerosol-based technology.
ground-state space group along with an a–b–c– octahedral tilt pattern. The
new system also favors thermodynamic cation nonstoichiometry due to
the calculated formation enthalpy of ΔHf = −148 meV/atom, lower than that of the pristine sample.12 The efficiency of KBaTeBiO6 is calculated
to be 0.057%, which is relatively low; however, it shows much better
stability than many other bismuth-based halide perovskites. Also,
solar cells composed of KBaTeBiO6 nanoparticles exhibit
a performance similar to those based on high-quality thin films of
other inorganic oxide perovskites. Much higher efficiency of the solar
cells based on KBaTeBiO6 can be achieved by controlling
and optimizing the stoichiometry and growing high-quality thin films
using aerosol-based technology.
Furthermore, studies have been carried out that effectively introduce pressure upon DPO materials in view of observing an enhanced properties regime such as inaccessible structural phase via temperature variation, Jahn–Teller distortions, band gap and photoluminescence evolution, as well as piezochromism.22 In recent advances, pressure-induced evaluation of various properties in DPOs is an exciting field in terms of its structural evolution and usage in various energy applications.
Transition-Metal-Based Double Perovskite Oxides
Transition-metal-based double perovskite oxides are those in which the B-site cation is a transition metal such as Ni and Fe. Along with a number of exotic phenomena such as room temperature magnetoresistance, magneto-capacitance, and magnetostriction, these transition-metal-based DPOs exhibit other thought-provoking properties such as metallic/half-metallic ferromagnetism and magnetoresistance, as observed in Sr2FeMoO6, which is a half-metallic ferromagnet,13 Sr2FeReO6, a half-metallic ferrimagnet,14 and Sr2FeWO6, an antiferromagnetic insulator.15
Theoretical studies
on lead-based DPOs, Pb2ScSbO6, have been reported
by Hnamte et al.24 Pb2ScSbO6 exhibits a face-centered cubic symmetry with a 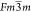 space group. The material shows a direct
band gap at 2.712 eV. This study also establishes the occurrence
of a large number of density states at both VB and CB edges along
with identifying the material as a semiconductor based on the band
structure and optical absorption study.
space group. The material shows a direct
band gap at 2.712 eV. This study also establishes the occurrence
of a large number of density states at both VB and CB edges along
with identifying the material as a semiconductor based on the band
structure and optical absorption study.
The tetragonal Sr2NiMoO6 (SNMO) has been studied by Xu et al.16 The structure studied shows an I4/m space group. Sr ions are located between two types of corner-sharing octahedra, NiO6 and MoO6. SNMO nanoparticles crystallize in work-like nanograins with 20–50 nm diameters. The SNMO DPO structure exhibits indirect transitions with a band gap of 2.1 eV. The band structure analysis reveals that the VB is dominated by Ni–3d and O–2p from the MoO6 octahedra, whereas the CB is dominated by Mo–5d and Ni–3d orbitals. Due to the position of the CB being more negative than the H2O reduction energy, SNMO may split H2O into H2 with irradiation of visible light. In addition, O2 evolution may also be observed since the VB is more positive than the O2 oxidation energy. The photoluminescence study on SNMO shows an average lifetime of the system to be 11.2 μs.16 This large carrier lifetime dictates the importance of Ni ions in delaying the recombination of excitons. A large carrier lifetime also serves as a benefit for the system to be used in photocatalysis. The photocatalytic activity in SNMO can be described from multivalent Ni2+/Ni3+ and Mo6+/Mo5+, which may contribute to improving the photodegradation. A schematic of multivalent Mo and Ni ions in Sr2NiMoO6 is shown in Figure 3. Ni3+ and Mo5+ ions in SNMO act as a donor and acceptor, respectively, altering the recombination of photocreated hole–electron pairs:
Figure 3.

Schematic representation of multivalent Mo and Ni ions in transition-metal-based double perovskite oxide, Sr2NiMoO6.
Rai et al. studied the magnetic properties of A2FeReO6 (A = Sr, Ba).17 With the analysis of density of states (DOS) and band structure in A2FeReO6 (A = Sr, Ba), they clearly observed the ground state of Sr-based Sr2FeReO6 to be a ferrimagnetic metal with the Fe d states contributing a VBM at 2.0 eV and a CBM at 2.2 eV. However, the Fe−d states in Ba-based Ba2FeReO6 possess a bandwidth of about 4.6 eV spread between −2.30 and 2.4 eV. Both systems show an insulating nature in the spin-up state with a finite band gap corresponding to the split between occupied Fe/Re 3d–t2g (dxy, dyz, dxz) and unoccupied Fe/Re 3d–eg (dx2–y2, dz2) with the majority contribution from Re−d and Fe−d orbitals in CB and VB, respectively. However, in the spin-down state, the band structure shows broader bands, indicating its metallic behavior. Along with the broad bands, it also shows the Fermi energy, EF, passing through the increasing peak, further establishing a confirmed spin-polarization at EF, which is a signature of half-metallic materials. Interband transitions along the Δ−H symmetry takes place in these indirect band gap materials. The metallic behavior of this material is further validated by optical absorption analysis in which reflection is more prominently observed.
Song et al. discussed the Bi2FeCrO6 structure and its magnetic properties.18 Various studies on Bi2FeCrO618,25,26 have shown that its ground-state structure exhibits a nonmetallic R−3 space group. The alternating rotations of O ions around Fe/Cr cations in FeO6/CrO6 octahedra, crystal field splitting, and spin exchange splitting lead the structure to displace from its cubic phase. The material has a semiconductor behavior with a band gap slightly larger than 0.26 eV formed between fully occupied Cr 3d–t2g and unoccupied Fe 3d–t2g bands. The magnetic order turns is ferrimagnetic with every Cr spin orienting upward and every Fe spin orienting downward. The Fe/Cr show high spin states along with a magnetic moment of 3.70μB for the Fe sphere and −2.18μB in case of the Cr sphere, indicating its antiparallel nature, along with each Fe3+ and Cr3+ cation contributing 5 and −3μB, respectively. The spin exchange interaction study of Bi2FeCrO6 among ferrimagnetic, ferromagnetic, and two magnetic orders obtained by reversing one of the two Cr/Fe spins in ferrimagnetic order was carried out by Song et al. The three magnetic structures, Fe–Cr, Fe–Fe, and Cr–Cr, have a ground-state energy of 310, 161, and 166 meV, respectively. The same work also supports the analysis of Curie temperature (TC) based on Monte Carlo simulations and has a reported Tc value slightly higher than 450 K, which is consistent with other experimentally reported high Curie temperature values above room temperature.27−31 However, few other works25,26 reported obtaining a low Curie temperature of ≈130 K.
Dutta et al.
reveals the study on Ba2(InM)O6 (M = Nb, Ta),19 whereas Ting et al. reported
Ba2(InNb)O6 (BIN) with a  crystal structure space group along with
the lattice parameter of a = 8.2819 Å.32,33 A work by Lufaso et al.34 emphasizes
the Ba2(InTa)O6 (BIT) system with a
crystal structure space group along with
the lattice parameter of a = 8.2819 Å.32,33 A work by Lufaso et al.34 emphasizes
the Ba2(InTa)O6 (BIT) system with a  space group and a lattice parameter of a = 8.2814 Å. However, Zurmühlen et al.
report infrared reflectivity on both BIN and BIT.35,36 The electronic property study on these materials shows that their
electrical properties are highly influenced by the interaction between
transition metal d orbitals and oxygen 2p orbitals. Yin et al.37 have done an extensive study on BIN with both
Raman scattering and UV–visible reflectance spectroscopy. In
the work by Yin et al.,37 different ionic
radii of cations leading to distortion in the octahedron was reported.
Three vibrational branches in the Raman spectra analogous to a stretching
mode, bending mode, and external mode further aid the justification
of octahedral distortion. The same work also establishes a report
with the band gap of BIN at 3.82 eV based on UV–visible
spectra. Work by Dutta et al. reveals the band structure of both BIN
and BIT to be similar. Both of these structures exhibit a direct band
gap at a high-symmetry Γ point. The band gaps of BIN and BIT
are reported to be 2.75 and 3.5 eV, respectively. A comparative
smaller band gap of BIN with BIT indicated the more covalent nature
of Nb–O bond compared to the Ta–O bond. The static dielectric
function (ε0) is reported to be 4.8 and 4.3 for BIN
and BIT, respectively.19 We can also establish
validation of the Penn model58 by comparing
the values of ε0 being inversely proportional to
the band gap, Eg. Based on optical function
analysis, the static refractive index (n0) is reported to be 2.2 and 2.1 for BIN and BIT, respectively.
space group and a lattice parameter of a = 8.2814 Å. However, Zurmühlen et al.
report infrared reflectivity on both BIN and BIT.35,36 The electronic property study on these materials shows that their
electrical properties are highly influenced by the interaction between
transition metal d orbitals and oxygen 2p orbitals. Yin et al.37 have done an extensive study on BIN with both
Raman scattering and UV–visible reflectance spectroscopy. In
the work by Yin et al.,37 different ionic
radii of cations leading to distortion in the octahedron was reported.
Three vibrational branches in the Raman spectra analogous to a stretching
mode, bending mode, and external mode further aid the justification
of octahedral distortion. The same work also establishes a report
with the band gap of BIN at 3.82 eV based on UV–visible
spectra. Work by Dutta et al. reveals the band structure of both BIN
and BIT to be similar. Both of these structures exhibit a direct band
gap at a high-symmetry Γ point. The band gaps of BIN and BIT
are reported to be 2.75 and 3.5 eV, respectively. A comparative
smaller band gap of BIN with BIT indicated the more covalent nature
of Nb–O bond compared to the Ta–O bond. The static dielectric
function (ε0) is reported to be 4.8 and 4.3 for BIN
and BIT, respectively.19 We can also establish
validation of the Penn model58 by comparing
the values of ε0 being inversely proportional to
the band gap, Eg. Based on optical function
analysis, the static refractive index (n0) is reported to be 2.2 and 2.1 for BIN and BIT, respectively.
There are several reported studies on Ba2MgWO6, among which Brik has reported a first-principles study,23 whereas the first experimental studies have been reported in another work38,39 which establishes the cubic phase of the material. The lattice constant of Ba2MgWO6 was found to be 8.016 Å.23,40 The system exhibited an indirect band gap of 2.89 eV based on a theoretical study,23 whereas it was reported to be 3.4 eV based on experimental observation.41 The band structure of Ba2MgWO6 obtained theoretically is shown in Figure 4. The bottom of the CB mainly consists of W–5d orbitals, and the top of VB is dominated by O–2p orbitals.23 The band structure analysis also reveals the presence of two sub-bands at the CB edge with a narrow gap of ≈1 eV. A lower band observed in the range of 3.5–4.5 eV is closely flat in nature, indicating an inferior electron mobility, whereas the upper band lying between 5.5 and 9.5 eV reveals a more dispersed nature. The bonding of Mg and Ba ions with the Ba2MgWO6 crystal lattice is expected to be ionic in nature,23 whereas that of composite WO6 is found to be mostly covalent due to the considerable difference between the calculated and formal charges of the W/O ions. The same work23 also reports the calculated refractive index (n0) as 2 for Ba2MgWO6. The density of states analysis establishes transition from O–2p to W–5d orbitals at ∼5 eV, whereas transition from the O–2p to Ba–5d orbital occurs at 9 eV. Distinct work on pressure effects in Ba2MgWO6 has been studied by Brik,23 which reveals the behavior of Ba2MgWO6 from a pressure of 0 to 30 GPa. The indication of pressure coefficient being 1 order of magnitude greater for the lattice constant than the W–O interatomic distance (0.0123 Å GPa–1 v/s 0.001 51 Å GPa–1) acknowledges the rigidity in W–O compressibility. As the pressure increases, there is an observed decrease in lattice constant, which is credited to the WO6 octahedra shifting closer to each other. The major ionic nature in the case of Ba–O and Mg–O causes these bonds to exhibit easy compressibility compared to that with the covalently interacting W–O bonds. Pressure coefficients for Ba–O and Mg–O distances are similar to the values of 0.00438 and 0.00464 Å GPa–1, respectively. Another important pressure manifestation in Ba2MgWO6 is the linear evolution of the band gap with a pressure coefficient of 0.006 01 eV GPa–1.
Figure 4.

Band structure of transition-metal-based double perovskite oxide, Ba2MgWO6 showing indirect band gap. Adapted with permission from ref (23). Copyright 2012 Elsevier.
Previous works on Sr2FeMO6 (M = Mo, W, Re)13−15 reveal the crystal structures of all three systems to be cubic in nature with Fe–M distances of 3.945, 3.975, and 3.945 Å, respectively. The same works also establish the FeM valence to be +8; that is, the number of d electrons per FeMo and FeW is found to be 6, whereas it is 7 in case of FeRe. In both ferromagnetic and antiferromagnetic states, five electrons fill and complete the majority of spin bands of Fe, while the minority of spin bands in both M = Mo, W contain one electron and M = Re holds two electrons. Further, the Curie temperatures of SFMO and SFRO materials are found to be 419 and 401 K, respectively.13,15 The cause for this large magnetic transition temperature was predicted by Sarma et al.,42 as the huge interatomic exchange coupling strength, J, between Fe and M and the low intra-atomic exchange (between M and M) strength I gives the robust intensified effective exchange at Mo due to the Fe 3d and Mo 4d hybridization. Both SFMO and SFRO may be considered as ferrimagnetic materials in nature since the magnetic moments of Fe in both the materials are aligned ferromagnetically, whereas Mo and Re exhibit induced magnetic moments which are coupled antiferromagnetically to Fe moments. However, these materials are categorized as ferromagnetic due to the fact that Mo and Re are nonmagnetic by their very nature and their antiparallel moments are induced by Fe moments via 4d/5d–3d hybridization. An interesting, yet contradicting, phenomenon of the antiferromagnetic nature with very low Néel temperature of 16–37 K occurs in a similar structure, Sr2FeWO6(SFWO), despite W being a 5d equivalent of Mo and occupying a position next to Re in the periodic table. Extrapolating Sarma’s prediction in the work,42 Kanamori et al. gave a generalized mechanism, now termed as Kanamori and Terakura mechanism,43 followed by Fang et al. who established a new mechanism on strong ferromagnetic stabilization in these materials.44 In this work, they successfully showed that stronger hybridization of O–2p with W–5d in the case of SFWO compared to that with the O–2p–Mo–4d hybridization in SFMO is the main source in lifting W 5d orbitals to higher energy, in turn paralyzing the ferromagnetic equalization. However, in the case of SFRO, the deeper Re–5d orbitals compared to W cancel the intensified p–d hybridization and rebuilds the ferromagnetic stabilization within the system.
Owing to its intensified magnetic properties, these transition-metal-based DPOs are suitable for spintronics. One could certainly look upon these type of materials for magnetization-related studies. It is a fascinating field to study the structural evolution of materials by application of external stimuli and its consequence on magnetic properties. The possibility of catalysis in these materials is also another view one could look upon.
Rare-Earth-Based Double Perovskite Oxides
Rare-earth (RE, also called as lanthanide)-based DPOs are of the form A2B′B″O6. This wider group is further classified into two subgroups based on the RE elemental occupancy site. One group falls under the RE element occupying the A-site in which the B′ and B″-site will be employed by transition-metal cations such as Ni or Mn. Another subgroup takes up the RE element at the B′- and/or B′′-site(s), whereas alkaline-earth metal such as Ba fits into the A-site. Recently, these DPOs have gained prominent interest among materialists due to their unique control over magnetic behavior, optical properties, and charge transfer.
RE Element Occupying the A-Site
The study by Sheikh et al.46 reports on RE-based DPO, RE2NiMnO6 (RE = La, Eu, Dy, Lu) (RENMO). Out of the four, three structures, ENMO, DMNO, and LuNMO show crystallization in the P21/m monoclinic phase, whereas LaNMO exhibits 44.36% crystallization in the P21/n monoclinic space group and 55.64% crystallization in the R3̅ rhombohedral space group. The Mn–O–Ni bond angle gradually decreases from 166.37° in LaNMO to 145.50° in LuNMO, accounting for an increased octahedral distortion and decreased tolerance factor. However, the average grain size of these structures increases from LaNMO to LuNMO in the order of 190 to 560 nm, respectively. The optical band gap of all four structures, RENMO (RE = La, Eu, Dy, Lu), are in a range similar to that of silicon (≈1.1 eV), showing their characteristics of being usable in photovoltaic applications. The observed band gap of LaNMO, ENMO, DNMO, and LuNMO is 1.08, 1.1, 1.19, and 1.12 eV, respectively. The analysis on charge carrier lifetime based on the frequency-dependent Bode plot in RENMO (RE = La, Eu, Dy, Lu) is reported to be 0.022, 0.110, 0.108, and 0.076 ms, respectively, establishing RENMO as useful for the photovoltaics industry. The same work46 also analyses the J–V characteristics of RENMO (RE = La, Eu, Dy, Lu). The combined effect of low series resistance (RS) and charge-transfer resistance (RCT) along with a high recombination resistance (Rrec) in LaNMO makes it the best candidate for solar cells among all four fabricated RENMO structures, with a 0.17% power conversion efficiency.
An interesting ferromagnetic material series, RE2NiMnO6 (RE = La, Pr, Sm, Tb) (RENMO), have been studied by Lekshmi et al.45 The polycrystalline RE2NiMnO6 (RE = La, Pr, Sm, Tb) has a P21/n monoclinic space group. In addition, there exists an observed decrease in lattice parameter, unit cell volume and bond angles in RENMO with a decrease in RE ionic radii. The unit cell parameter including cell volume and bond angle with respect to RE ionic radii is shown in Figure 5a. The study involving thermo-magnetization for the RE2NiMnO6 (RE = La, Pr, Sm, Tb) compound has been reported,45 wherein the material magnetization was reported with a temperature range of 10–300 K. It is interesting to know that all four compounds show paramagnetic to ferromagnetic transitions at higher temperature (>100 K). Sm2NMO and Tb2NMO exhibit a magnetic transition at 160 and 110 K, respectively. This is followed by a re-entrant magnetic transition at a low temperature of 20 and 12 K, respectively. The presence of inherent coupling of the Ni–Mn network along with the RE3+ ion47,48 might be the probable cause of the low-temperature re-entrant nature.49 A decrease in ionic radii from LaNMO to TbNMO also contributes to the systematic decrease in their Curie temperature (TC). However, Tmax and Eα1 show an indirect proportionality with ionic radii of RE, indicating that electron hopping between Ni and Mn is more suppressed with decreasing RE ionic radii. Variation of Tmax with respect to ionic radii is shown in Figure 5b. The tendency of coupling between magnetic and dielectric properties of RENMO implies a spin–lattice coupling, opening more research areas in spintronics. However, the double transition at ≈160 and ≈240 K reported for disordered La2NiMnO6 makes it an interesting compound. Even though highly ordered La2NiMnO6 exhibits a magnetic transition only at a high temperature of 270 K,50 its disordered phase also exhibits a magnetic transition at a relatively lower temperature of ≈150 K due to the strong superexchange interaction between Ni3+–O–Mn3+ bonds. This unclear nature of two magnetic transitions in La2NiMnO6 further opens up several research opportunities in this direction.
Figure 5.

(a) Representation of the change in unit cell parameter including cell volume and bond angle with RE ionic radii. (b) Variation of Tmax of tan δ(T) and Eα1 with RE ionic radii. Inset: Evolution of magnetic TC with RE ionic radii. Adapted with permission from ref (45). Copyright 2014 Elsevier.
RE Element Occupying the B-Site
The electrical transport
mechanism in Ba2ErNbO6 (BEN) was reported by
Mukherjee et al.51 The cubic perovskite
BEN crystallizes in the 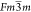 space group along with a tolerance factor
value of Tf = 0.98, furthermore establishing
its cubic structure. The average grain size of BEN is ≈1.5 μm
via scanning electron microscopy. ErO6 and NbO6 octahedra in BEN do not show tilting in any direction, which can
be represented as a0a0a0 in Glazer notation.52−54 Two well-defined bands found in the infrared (IR) spectrum vibrational
mode at 387 and 600 cm–1 represent asymmetric
bending and asymmetric stretching of NbO6 octahedron, respectively,
whereas a weak band at 846 cm–1 is due to
the symmetric stretching in the NbO6 octahedra. In addition,
a dip in the IR region’s vibrational mode at 418 cm–1 is observed, corresponding to the stretching of ErO6 octahedra. Further, the work also reports the activation
energy of BEN as Ea = 0.85 eV along
with the relaxation time corresponding to the frequency-dependent
dielectric loss obeying the Arrhenius law.
space group along with a tolerance factor
value of Tf = 0.98, furthermore establishing
its cubic structure. The average grain size of BEN is ≈1.5 μm
via scanning electron microscopy. ErO6 and NbO6 octahedra in BEN do not show tilting in any direction, which can
be represented as a0a0a0 in Glazer notation.52−54 Two well-defined bands found in the infrared (IR) spectrum vibrational
mode at 387 and 600 cm–1 represent asymmetric
bending and asymmetric stretching of NbO6 octahedron, respectively,
whereas a weak band at 846 cm–1 is due to
the symmetric stretching in the NbO6 octahedra. In addition,
a dip in the IR region’s vibrational mode at 418 cm–1 is observed, corresponding to the stretching of ErO6 octahedra. Further, the work also reports the activation
energy of BEN as Ea = 0.85 eV along
with the relaxation time corresponding to the frequency-dependent
dielectric loss obeying the Arrhenius law.
The structural study
on Ba2RESbO6 (RE = Er, Ho) was carried out by
Halder et al.55 Both Ba2ErSbO6 (BES) and Ba2HoSbO6 (BHS) systems were
found to have cubic symmetry with a 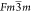 space group. Lattice parameters of BES
and BHS were 8.4036 and 8.3908 Å, respectively. Density
of states analysis55 shows that electronic
properties of both BES and BHS are governed by the interaction between
transition metal d orbitals, rare-earth ion f orbitals, and oxygen
2p orbitals. Interestingly, both BES and BHS exhibit similar band
profiles. BES and BHS possess a direct band gap along the highly symmetric
Γ–Γ points with a band gap value of 2.2 and 3.45
eV, respectively. The desired nature of direct Γ–Γ
transitions gives these materials potential in the optoelectronics
industry for fabricating laser diodes and light-emitting diodes in
visible and ultraviolet regions. The static refractive index n0 for BES and BHS was found to be 1.87 and 2.11,
respectively, whereas the calculated static dielectric constant ε(0)
was found to be 3.48 and 4.52, respectively. Based on the Born effective
charge (BEC) analysis,55 we can clearly
see larger BEC values for rare earth elements (Er, Ho) and Sb ions,
indicating its behavior as a donor, whereas the smaller BEC value
in case of O ions indicates its behavior as an acceptor, due to which
the charge transfer occurs from Ho, Er, or Sb to O ions.
space group. Lattice parameters of BES
and BHS were 8.4036 and 8.3908 Å, respectively. Density
of states analysis55 shows that electronic
properties of both BES and BHS are governed by the interaction between
transition metal d orbitals, rare-earth ion f orbitals, and oxygen
2p orbitals. Interestingly, both BES and BHS exhibit similar band
profiles. BES and BHS possess a direct band gap along the highly symmetric
Γ–Γ points with a band gap value of 2.2 and 3.45
eV, respectively. The desired nature of direct Γ–Γ
transitions gives these materials potential in the optoelectronics
industry for fabricating laser diodes and light-emitting diodes in
visible and ultraviolet regions. The static refractive index n0 for BES and BHS was found to be 1.87 and 2.11,
respectively, whereas the calculated static dielectric constant ε(0)
was found to be 3.48 and 4.52, respectively. Based on the Born effective
charge (BEC) analysis,55 we can clearly
see larger BEC values for rare earth elements (Er, Ho) and Sb ions,
indicating its behavior as a donor, whereas the smaller BEC value
in case of O ions indicates its behavior as an acceptor, due to which
the charge transfer occurs from Ho, Er, or Sb to O ions.
Rare-earth-based DPOs have applications in various fields such as optoelectronic and photovoltaic industries. A huge scope is oriented toward the study of magnetic transitions in these materials, which could be established in the field of spintronics. Many unknown attributes underlying magnetic phenomena disclose several new opportunities for materialists to look into.
Mixed Cationic/Anionic Double Perovskite Oxides
Apart from pure DPOs, mixed cationic/anionic DPOs have emerged as powerful materials that exhibit interesting properties which are highly influential in achieving usable materials as photoanodes in photochemical and photoelectrochemical water splitting as well as in spintronics.
Analysis by Kangsabanik et al.56 not only reveals the understanding of new Na2BIO6 (B = Bi, In) DPOs but also constructs the orbital engineering approach for band gap tuning. Interesting structural observation in ANaIO6 (A = Sr, Ca, Pb), where octahedral distortion is due to the Na atomic radii that lies exactly between that of Sr and Ca, both of which possess a monoclinic P21/n space group that can also be seen in same work. From the structural analysis, among all of the structures, only Na2BIO6 (B = Bi, In) is reported to be stable in the monoclinic P21/n phase. The highly covalent nature of Bi–O bonding in Na2BiIO6 (NBIO) compared to that of In–O in Na2InIO6 (NIIO) leads to higher orbital overlap in the case of NBIO, giving rise to more dispersive valence bands. NBIO possess an indirect band gap of 2.33 eV at highly symmetric C and A points for VBM and CBM, respectively, whereas NIIO has a direct band gap of 2.48 eV at the highly symmetric Γ point. In further analysis, Bi substitution in the case of NIIO (Na2Bi0.25In0.75IO6) observes the introduction of hybridization between lone pair Bi 6s and O 2p orbitals, which, in turn, establishes the reduction in band gap to 1.66 eV, as shown in Figure 6. This new Bi-substituted NIIO compound possesses both an ideal band gap as well as a dispersive band structure that is essential for photovoltaic applications. Although Tl is toxic, it is substituted for NBIO mainly to validate the orbital-engineering method. This new compound, Na2Tl0.25Bi0.75IO6, exhibits an intermediate band below the CBM consisting of Tl s states, helping to reduce the indirect band gap for 1.78 eV, shown in Figure 6. Better absorption characteristics and dispersed band-edges make these compounds applicable in the photovoltaics industry, whereas the position of the VBM, which is the occurrence of oxygen evolution reaction (OER), efficiently makes the materials useful as photoanodes in photochemical and photoelectrochemical (PEC) water splitting. Promising optical absorption, highly dispersive band-edges, and well positioned VBM for efficient OER make the substituted compounds potential candidates for PC/PEC applications.
Figure 6.
Band structure and optical transition probability (p2) between an allowed band at the minimum band gap in (a) Na2Bi0.25In0.75IO6 and (b) Na2Tl0.25Bi0.75IO6. (c) Representation of molecular orbitals for Na2Bi0.25In0.75IO6, where empty and filled boxes specify unoccupied conduction bands and occupied valence bands. (d) Computed absorption coefficient spectra. (e) Spectroscopic limited maximum efficiency versus thickness of the material at 298 K for pristine and mixed compounds. (f) Band-edge positions with respect to water redox levels for Na2BIO6 (B = Bi, In, In0.25Bi0.75) marked on the x-axis as Tl0.25Bi0.75 (TlBi), Bi0.25In0.75 (BiIn), Tl0.25In0.75 (TlIn). Adapted from ref (56). Copyright 2020 American Chemical Society..
A family of DPOs, A2BB′O6 (A = Ba,
Sr) (BB′ = FeRe, MnMo, MnRe) were studied by Ali et al.57 Interestingly, all of these DPOs exhibit a cubic
structure with the 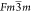 space group at ambient temperature. The
bond lengths calculated between B–O and B′–O
indicate that the B′O6 octahedra are marginally
smaller than the BO6 octahedra. Analysis by Ali et al.57 on A2BB′O6 (A =
Ba, Sr) (BB′ = FeRe, MnMo, MnRe) reported both a generalized
gradient approximation (GGA) approach as well as a GGA+U approach.
The GGA-based approach showed d–d hybridization between FeRe,
MnMo, and MnRe in both spin-up and spin-down states, indicating the
metallic nature of all of these compounds; the GGA+U-based approach
emphasized only Ba2FeReO6, Sr2MnMoO6, and Sr2MnReO6 to be metallic, whereas
Ba2MnReO6, Ba2MnMoO6,
and Sr2FeReO6 to be half-metallic. Spin−orbit
coupling (SOC), including a GGA+U approach, discovers further interesting
observations that indicate all the structures to be metallic in nature.
Since SOC becomes a crucial factor to be considered during the presence
of heavy elements such as Ba and Re, we may conclude that the results
based on SOC included GGA+U; that is, all structures showing a metallic
nature were more accurate. The Fermi region of all of the structures
is mainly occupied by BB′ ions, which is mainly d state orbitals.
The study on charge density by Ali et al.57 reveals the covalent bonding between B/B′ with O atoms driven
by the strong hybridization between cationic d orbitals and anionic
p orbitals, whereas the ionic bond existing between Mn and O has been
studying based on its electronegativity. The same study also shows
that the ionic nature of O with alkaline earth metal decreases from
Ba to Sr in A2BB′O6 (A = Ba, Sr) (BB′
= FeRe, MnMo, MnRe). In addition, all of the considered systems in
A2BB′O6 (A = Ba, Sr) (BB′ = FeRe,
MnMo, MnRe) exhibit ferromagnetic behavior with high magnetic moments.
space group at ambient temperature. The
bond lengths calculated between B–O and B′–O
indicate that the B′O6 octahedra are marginally
smaller than the BO6 octahedra. Analysis by Ali et al.57 on A2BB′O6 (A =
Ba, Sr) (BB′ = FeRe, MnMo, MnRe) reported both a generalized
gradient approximation (GGA) approach as well as a GGA+U approach.
The GGA-based approach showed d–d hybridization between FeRe,
MnMo, and MnRe in both spin-up and spin-down states, indicating the
metallic nature of all of these compounds; the GGA+U-based approach
emphasized only Ba2FeReO6, Sr2MnMoO6, and Sr2MnReO6 to be metallic, whereas
Ba2MnReO6, Ba2MnMoO6,
and Sr2FeReO6 to be half-metallic. Spin−orbit
coupling (SOC), including a GGA+U approach, discovers further interesting
observations that indicate all the structures to be metallic in nature.
Since SOC becomes a crucial factor to be considered during the presence
of heavy elements such as Ba and Re, we may conclude that the results
based on SOC included GGA+U; that is, all structures showing a metallic
nature were more accurate. The Fermi region of all of the structures
is mainly occupied by BB′ ions, which is mainly d state orbitals.
The study on charge density by Ali et al.57 reveals the covalent bonding between B/B′ with O atoms driven
by the strong hybridization between cationic d orbitals and anionic
p orbitals, whereas the ionic bond existing between Mn and O has been
studying based on its electronegativity. The same study also shows
that the ionic nature of O with alkaline earth metal decreases from
Ba to Sr in A2BB′O6 (A = Ba, Sr) (BB′
= FeRe, MnMo, MnRe). In addition, all of the considered systems in
A2BB′O6 (A = Ba, Sr) (BB′ = FeRe,
MnMo, MnRe) exhibit ferromagnetic behavior with high magnetic moments.
The study of hybrid materials has always been exciting in glazing intermediate regimes, and these mixed cations/anions definitely serve as one such group. With the interplay of cation/anion composition space, much more advanced phenomena with respect to all electronic, optical, and magnetic properties are expected. Further studies on these materials are in the limelight for establishing much better materials with flexibility of structural deformation along with use in various applicational fields such as catalysis and water splitting and is certainly an open field to seek new materials.
Future Outlook and Summary
In this perspective, we have discussed how to exploit the double perovskite oxides and their derivatives for widespread applications and provided an outlook on the challenges and opportunities that lie ahead for this enticing class of compounds. We posit that this class of materials has enormous potential for the future development of multidisciplinary research combining spintronics, electronics, and photonics. This perspective attempts to describe how the properties of DPOs are tuned for specific applications. Compositional tuning and band gap engineering are extensively used techniques due to the flexibility of the perovskite structure. Though a lot of research has already been done on bulk perovskite, layered and heterostructure-combined perovskites have not been thoroughly looked at by the scientific community. Without distorting the advantages of oxide perovskite structures and properties, we have addressed how the shortcomings of corresponding applications are now a major challenge for the scientific community. An extensive approach involving passivation, intrinsic material stability, and doping strategies needs to be adopted to overcome the shortcomings of DPOs.
The synergistic effect in terms of electronic and optical properties of the individual double perovskite oxides could be amplified further when the corresponding heterostructure can be constructed from them. In this regard, theoretical insight would be influential to predict new combinations of DPOs for efficient heterostructure material. The theoretical investigation also facilitates resolving lattice mismatch in individual oxide perovskites for heterostructure construction. Another route for efficient and novel DPOs is through exerting hydrostatic pressure. This would certainly pave the way to find new compositions and stoichiometries that might be difficult to synthesize in laboratory. The electronic and optical properties can be tuned under the influence of external strain, and researchers can further try to expand the possibility of finding new stable and good-performing materials which could later be synthesized and put into practical use. One can also perform a systematic high-throughput screening based on electronic and optical properties to find a promising double perovskite oxide material for applications in photovoltaics and photocatalysis. Magnetic properties are known to be seen in oxide perovskites, and DPOs have already proven to have varied magnetic properties. Transition-metal-based DPOs are exceptionally well-suited materials for the study of magnetic behavior and for further applications in spintronics. DPOs span various categories both in terms of understanding fundamental phenomena as well as in application-oriented tasks and, thus, create immense research opportunities. Overall, there is a huge potential in the double perovskite oxide family that can be manifested further while tuning the underlying electronic and optical properties.
Acknowledgments
M.G.B. and S.C. are thankful to the institute PhD fellowship of HRI and HBNI. S.C. would like to acknowledge HRI Prayagraj and DST-SERB Funding (SRG/2020/001707) for the infrastructure and funding.
Author Contributions
CRediT: Manasa G Basavarajappa writing-original draft (equal), writing-review & editing (equal); Sudip Chakraborty conceptualization (lead), supervision (equal), writing-original draft (equal), writing-review & editing (equal).
The authors declare no competing financial interest.
References
- Goldschmidt V. M. Die Gesetze der Krystallochemie. Die Naturwissenschaften 1926, 14, 477–485. 10.1007/BF01507527. [DOI] [Google Scholar]
- Hoskin P. W. O. In Minerals: Their Constitution and Origin; Wenk H.-R., Bulakh A. G., Eds.; Cambridge University Press: Cambridge, U.K., 2004. [Google Scholar]
- Szuromi P.; Grocholski B. Natural and engineered perovskites. Science 2017, 358, 732–733. 10.1126/science.358.6364.732. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Singh S. Improved structure stability, optical and magnetic properties of Ca and Ti co-substituted BiFeO3 nanoparticles. Appl. Surf. Sci. 2016, 386, 78–83. 10.1016/j.apsusc.2016.05.163. [DOI] [Google Scholar]
- Patterson F. K.; Moeller C. W.; Ward R. Magnetic Oxides of Molybdenum(V) and Tungsten(V) with the Ordered Perovskite Structure. Inorg. Chem. 1963, 2, 196–198. 10.1021/ic50005a050. [DOI] [Google Scholar]
- Anderson M.; Greenwood K.; Taylor G.; Poeppelmeier K. B-cation arrangements in double perovskites. Prog. Solid State Chem. 1993, 22, 197–233. 10.1016/0079-6786(93)90004-B. [DOI] [Google Scholar]
- Zhao F.; Yue Z.; Gui Z.; Li L. Preparation, Characterization and Microwave Dielectric Properties of A2BWO6(A = Sr, Ba ; B = Co, Ni, Zn) Double Perovskite Ceramics. Jpn. J. Appl. Phys. 2005, 44, 8066–8070. 10.1143/JJAP.44.8066. [DOI] [Google Scholar]
- Barnes P. W.; Lufaso M. W.; Woodward P. M. Structure determination of A2M3+TaO6 and A2M3+NbO6 ordered perovskites: octahedral tilting and pseudosymmetry. Acta Crystallographica Section B Structural Science 2006, 62, 384–396. 10.1107/S0108768106002448. [DOI] [PubMed] [Google Scholar]
- Vasala S.; Karppinen M. A2B’B”O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. 10.1016/j.progsolidstchem.2014.08.001. [DOI] [Google Scholar]
- Yin W.-J.; Weng B.; Ge J.; Sun Q.; Li Z.; Yan Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. 10.1039/C8EE01574K. [DOI] [Google Scholar]
- Roth R.; Vanderah T.; Bordet P.; Grey I.; Mumme W.; Cai L.; Nino J. Pyrochlore formation, phase relations, and properties in the CaO–TiO2–(Nb,Ta)2O5 systems. J. Solid State Chem. 2008, 181, 406–414. 10.1016/j.jssc.2007.12.005. [DOI] [Google Scholar]
- Thind A. S.; Kavadiya S.; Kouhnavard M.; Wheelus R.; Cho S. B.; Lin L.-Y.; Kacica C.; Mulmudi H. K.; Unocic K. A.; Borisevich A. Y.; Pilania G.; Biswas P.; Mishra R. KBaTeBiO6: A Lead-Free, Inorganic Double-Perovskite Semiconductor for Photovoltaic Applications. Chem. Mater. 2019, 31, 4769–4778. 10.1021/acs.chemmater.9b01025. [DOI] [Google Scholar]
- Kobayashi K.-I.; Kimura T.; Sawada H.; Terakura K.; Tokura Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. 10.1038/27167. [DOI] [Google Scholar]
- Kobayashi K.-I.; Kimura T.; Tomioka Y.; Sawada H.; Terakura K.; Tokura Y. Intergrain tunneling magnetoresistance in polycrystals of the ordered double perovskiteSr2FeReO6. Phys. Rev. B 1999, 59, 11159–11162. 10.1103/PhysRevB.59.11159. [DOI] [Google Scholar]
- Kobayashi K.-I.; Kimura T.; Tomioka Y.; Sawada H.; Terakura K.; Tokura Y. Intergrain tunneling magnetoresistance in polycrystals of the ordered double perovskiteSr2FeReO6. Phys. Rev. B 1999, 59, 11159–11162. 10.1103/PhysRevB.59.11159. [DOI] [Google Scholar]
- Xu L.; Wan Y.; Xie H.; Huang Y.; Yang L.; Qin L.; Seo H. J. Synthesis, surface structure and optical properties of double perovskite Sr2NiMoO6 nanoparticles. Appl. Surf. Sci. 2016, 389, 849–857. 10.1016/j.apsusc.2016.07.117. [DOI] [Google Scholar]
- Rai D.; Shankar A.; Ghimire M.; Sandeep; Thapa R. The electronic, magnetic and optical properties of double perovskite A2FeReO6 (A = Sr, Ba) from first principles approach. Comput. Mater. Sci. 2015, 101, 313–320. 10.1016/j.commatsci.2015.01.027. [DOI] [Google Scholar]
- Song Z.-W.; Liu B.-G. Electronic structure and magnetic and optical properties of double perovskite Bi2FeCrO6from first-principles investigation. Chinese Physics B 2013, 22, 047506. 10.1088/1674-1056/22/4/047506. [DOI] [Google Scholar]
- Dutta A.; Sinha T. First principles study of electronic structure and optical properties of double perovskite Ba2(InM)O6 [, Ta]. Solid State Commun. 2010, 150, 1173–1177. 10.1016/j.ssc.2010.04.023. [DOI] [Google Scholar]
- Sarangi A.; Basavarajappa M. G.; Chakraborty S. Tuning composition space in lead-free divalent and tetravalent halide perovskite: a critical review. Emergent Materials 2021, 1–12. 10.1007/s42247-021-00297-0.33718776 [DOI] [Google Scholar]
- Modeshia D. R.; Walton R. I. Solvothermal synthesis of perovskites and pyrochlores: crystallisation of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 39, 4303. 10.1039/b904702f. [DOI] [PubMed] [Google Scholar]
- Basavarajappa M. G.; Nazeeruddin M. K.; Chakraborty S. Evolution of hybrid organic–inorganic perovskite materials under external pressure. Applied Physics Reviews 2021, 8, 041309. 10.1063/5.0053128. [DOI] [Google Scholar]
- Brik M. First-principles calculations of electronic, optical and elastic properties of Ba2MgWO6 double perovskite. J. Phys. Chem. Solids 2012, 73, 252–256. 10.1016/j.jpcs.2011.10.034. [DOI] [Google Scholar]
- Hnamte L.; Joshi H.; Rai D.; Thapa R. Electronic and optical properties of double perovskite oxide Pb2ScMO6 (M = Ta, Sb) using a first principles approach. J. Phys. Chem. Solids 2019, 129, 188–195. 10.1016/j.jpcs.2019.01.004. [DOI] [Google Scholar]
- Baettig P.; Spaldin N. A. Ab initio prediction of a multiferroic with large polarization and magnetization. Appl. Phys. Lett. 2005, 86, 012505. 10.1063/1.1843290. [DOI] [Google Scholar]
- Baettig P.; Ederer C.; Spaldin N. A. First principles study of the multiferroicsBiFeO3,Bi2FeCrO6, andBiCrO3: Structure, polarization, and magnetic ordering temperature. Phys. Rev. B 2005, 72, 214105. 10.1103/PhysRevB.72.214105. [DOI] [Google Scholar]
- Nechache R.; Harnagea C.; Pignolet A.; Normandin F.; Veres T.; Carignan L.-P.; Ménard D. Growth, structure, and properties of epitaxial thin films of first-principles predicted multiferroic Bi2FeCrO6. Appl. Phys. Lett. 2006, 89, 102902. 10.1063/1.2346258. [DOI] [Google Scholar]
- Kamba S.; Nuzhnyy D.; Nechache R.; Závěta K.; Nižňanský D.; Šantavá E.; Harnagea C.; Pignolet A. Infrared and magnetic characterization of multiferroic Bi2FeCrO6 thin films over a broad temperature range. Phys. Rev. B 2008, 77, 104111. 10.1103/PhysRevB.77.104111. [DOI] [Google Scholar]
- Nechache R.; Harnagea C.; Carignan L.-P.; Gautreau O.; Pintilie L.; Singh M. P.; Ménard D.; Fournier P.; Alexe M.; Pignolet A. Epitaxial thin films of the multiferroic double perovskite Bi2FeCrO6 grown on (100)-oriented SrTiO3 substrates: Growth, characterization, and optimization. J. Appl. Phys. 2009, 105, 061621. 10.1063/1.3073826. [DOI] [Google Scholar]
- Aïssa B.; Nechache R.; Therriault D.; Rosei F.; Nedil M. High-frequency electromagnetic properties of epitaxial Bi2FeCrO6 thin films grown by pulsed laser deposition. Appl. Phys. Lett. 2011, 99, 183505. 10.1063/1.3657528. [DOI] [Google Scholar]
- Nechache R.; Harnagea C.; Pignolet A. Multiferroic properties—structure relationships in epitaxial Bi2FeCrO6thin films: recent developments. J. Phys.: Condens. Matter 2012, 24, 096001. 10.1088/0953-8984/24/9/096001. [DOI] [PubMed] [Google Scholar]
- Ting V.; Liu Y.; Withers R.; Krausz E. An electron diffraction and bond valence sum study of the space group symmetries and structures of the photocatalytic 1:1 ordered A2InNbO6 double perovskites (A = Ca2+, Sr2+, Ba2+). J. Solid State Chem. 2004, 177, 979. 10.1016/j.jssc.2003.09.040. [DOI] [Google Scholar]
- Ting V.; Liu Y.; Withers R.; Norén L.; James M.; Fitz Gerald J. A structure and phase analysis investigation of the “1:1” ordered A2InNbO6 perovskites (A = Ca2+, Sr2+, Ba2+). J. Solid State Chem. 2006, 179, 551–562. 10.1016/j.jssc.2005.11.012. [DOI] [Google Scholar]
- Lufaso M. W.; Barnes P. W.; Woodward P. M. Structure prediction of ordered and disordered multiple octahedral cation perovskites using SPuDS. Acta Crystallographica Section B Structural Science 2006, 62, 397–410. 10.1107/S010876810600262X. [DOI] [PubMed] [Google Scholar]
- Zurmühlen R.; Petzelt J.; Kamba S.; Voitsekhovskii V. V.; Colla E.; Setter N. Dielectric spectroscopy of Ba(B1/2’B1/2‘)O3complex perovskite ceramics: Correlations between ionic parameters and microwave dielectric properties. I. Infrared reflectivity study (1012–1014 Hz). J. Appl. Phys. 1995, 77, 5341–5350. 10.1063/1.359597. [DOI] [Google Scholar]
- Zurmühlen R.; Petzelt J.; Kamba S.; Kozlov G.; Volkov A.; Gorshunov B.; Dube D.; Tagantsev A.; Setter N. Dielectric spectroscopy of Ba(B1/2’B1/2‘)O3complex perovskite ceramics: Correlations between ionic parameters and microwave dielectric properties. II. Studies below the phonon eigenfrequencies (102–1012 Hz). J. Appl. Phys. 1995, 77, 5351–5364. 10.1063/1.359290. [DOI] [Google Scholar]
- Yin J.; Zou Z.; Ye J. Photophysical and photocatalytic properties of the visible-light-driven photocatalysts BaIn0.5Nb0.5O3, BaCo1/3Nb2/3O3 and BaNi1/3Nb2/3O3. Res. Chem. Intermed. 2005, 31, 463–475. 10.1163/1568567053956707. [DOI] [Google Scholar]
- Penn D. R. Wave-Number-Dependent Dielectric Function of Semiconductors. Phys. Rev. 1962, 128, 2093. 10.1103/PhysRev.128.2093. [DOI] [Google Scholar]
- Meenakshi S.; Vijayakumar V.; Achary S.; Tyagi A. High pressure investigation on double perovskite Ba2MgWO6. J. Phys. Chem. Solids 2011, 72, 609–612. 10.1016/j.jpcs.2011.01.012. [DOI] [Google Scholar]
- Steward E. G.; Rooksby H. P. Pseudo-cubic alkaline-earth tungstates and molybdates of theR3MX6type. Acta Crystallogr. 1951, 4, 503–507. 10.1107/S0365110X51001719. [DOI] [Google Scholar]
- Pychlau P. Another method of calibration for low energy X-ray beams. Phys. Med. Biol. 1974, 19, 386–386. 10.1088/0031-9155/19/3/011. [DOI] [PubMed] [Google Scholar]
- Eng H. W.; Barnes P. W.; Auer B. M.; Woodward P. M. Investigations of the electronic structure of d0 transition metal oxides belonging to the perovskite family. J. Solid State Chem. 2003, 175, 94–109. 10.1016/S0022-4596(03)00289-5. [DOI] [Google Scholar]
- Sarma D. D.; Mahadevan P.; Saha-Dasgupta T.; Ray S.; Kumar A. Electronic Structure ofSr2FeMoO6. Phys. Rev. Lett. 2000, 85, 2549–2552. 10.1103/PhysRevLett.85.2549. [DOI] [PubMed] [Google Scholar]
- Kanamori J.; Terakura K. A General Mechanism Underlying Ferromagnetism in Transition Metal Compounds. J. Phys. Soc. Jpn. 2001, 70, 1433–1434. 10.1143/JPSJ.70.1433. [DOI] [Google Scholar]
- Fang Z.; Terakura K.; Kanamori J. Strong ferromagnetism and weak antiferromagnetism in double perovskites:Sr2FeMO6(M = Mo,W, and Re). Phys. Rev. B 2001, 63, 180407. 10.1103/PhysRevB.63.180407. [DOI] [Google Scholar]
- Lekshmi P. N.; Vasundhara M.; Varma M. R.; Suresh K.; Valant M. Structural, magnetic and dielectric properties of rare earth based double perovskites RE2NiMnO6 (RE = La, pr, Sm, Tb). Physica B: Condensed Matter 2014, 448, 285–289. 10.1016/j.physb.2014.04.057. [DOI] [Google Scholar]
- Sheikh M. S.; Ghosh D.; Dutta A.; Bhattacharyya S.; Sinha T. Lead free double perovskite oxides Ln2NiMnO 6 (Ln = La, Eu, Dy, Lu), a new promising material for photovoltaic application. Materials Science and Engineering: B 2017, 226, 10–17. 10.1016/j.mseb.2017.08.027. [DOI] [Google Scholar]
- Booth R.; Fillman R.; Whitaker H.; Nag A.; Tiwari R.; Ramanujachary K.; Gopalakrishnan J.; Lofland S. An investigation of structural, magnetic and dielectric properties of R2NiMnO6 (R = rare earth, Y). Mater. Res. Bull. 2009, 44, 1559–1564. 10.1016/j.materresbull.2009.02.003. [DOI] [Google Scholar]
- Yang W. Z.; Liu X. Q.; Zhao H. J.; Lin Y. Q.; Chen X. M. Structure, magnetic, and dielectric characteristics of Ln2NiMnO6 (Ln = Nd and Sm) ceramics. J. Appl. Phys. 2012, 112, 064104. 10.1063/1.4752262. [DOI] [Google Scholar]
- Lekshmi P. N.; Raji G. R.; Vasundhara M.; Varma M. R.; Pillai S. S.; Valant M. Re-entrant spin glass behaviour and magneto-dielectric effect in insulating Sm2NiMnO6 double perovskite. Journal of Materials Chemistry C 2013, 1, 6565. 10.1039/c3tc31203h. [DOI] [Google Scholar]
- Rogado N. S.; Li J.; Sleight A. W.; Subramanian M. A. Magnetocapacitance and Magnetoresistance Near Room Temperature in a Ferromagnetic Semiconductor: La2NiMnO6. Adv. Mater. 2005, 17, 2225–2227. 10.1002/adma.200500737. [DOI] [Google Scholar]
- Mukherjee R.; Ghosh B.; Saha S.; Bharti C.; Sinha T. Structural and electrical transport properties of a rare earth double perovskite oxide: Ba2ErNbO6. Journal of Rare Earths 2014, 32, 334–342. 10.1016/S1002-0721(14)60076-4. [DOI] [Google Scholar]
- Glazer A. M. The classification of tilted octahedra in perovskites. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1972, 28, 3384–3392. 10.1107/S0567740872007976. [DOI] [Google Scholar]
- Woodward P. M. Octahedral Tilting in Perovskites. I. Geometrical Considerations. Acta Crystallographica Section B Structural Science 1997, 53, 32–43. 10.1107/S0108768196010713. [DOI] [PubMed] [Google Scholar]
- Woodward P. M. Octahedral Tilting in Perovskites. II. Structure Stabilizing Forces. Acta Crystallographica Section B Structural Science 1997, 53, 44–66. 10.1107/S0108768196012050. [DOI] [Google Scholar]
- Halder S.; Mukherjee R.; Dutta A.; Sinha T. P. Exploring the electronic structure and optical properties of double perovskite Ba2RESbO6 (RE = Ho, Er) from first-principles calculations. Ferroelectrics 2017, 518, 163–170. 10.1080/00150193.2017.1360650. [DOI] [Google Scholar]
- Kangsabanik J.; Alam A. Ab Initio Discovery of Stable Double Perovskite Oxides Na2BIO6 (B = Bi, In) with Promising Optoelectronic Properties. J. Phys. Chem. Lett. 2020, 11, 5148–5155. 10.1021/acs.jpclett.0c01256. [DOI] [PubMed] [Google Scholar]
- Ali S.; Khan W.; Murtaza G.; Yaseen M.; Ramay S. M.; Mahmood A. First principles study of magnetic and electronic properties of A 2 BB′O 6 (A = Ba, Sr) (BB′ = FeRe, MnMo, and MnRe) double perovskites. J. Magn. Magn. Mater. 2017, 441, 113–123. 10.1016/j.jmmm.2017.05.035. [DOI] [Google Scholar]




