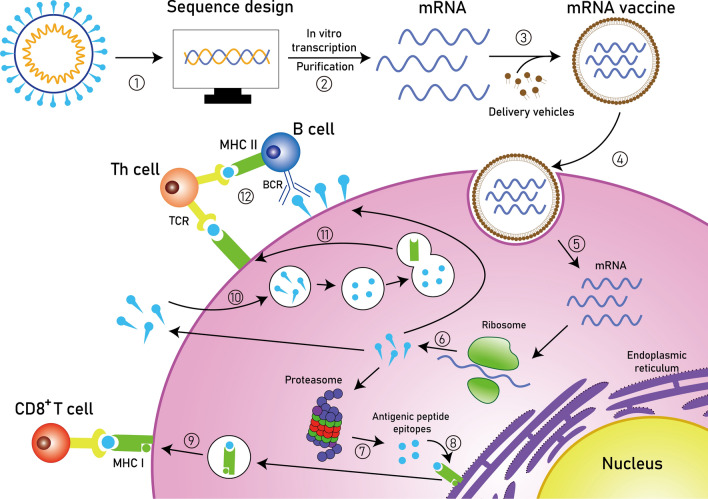
Fig. 2.
Principles of synthetic mRNA pharmacology. Step 1: After obtaining the pathogen genome, the target antigen sequence is designed and then inserted into the plasmid DNA vector. Step 2: The linearized plasmid DNA template is used for in vitro transcription and the resulting synthetic mRNA is purified. Step 3: The mRNA vaccine is prepared by encapsulating the purified mRNA with delivery vehicles. Step 4: The mRNA vaccine is taken up by endocytosis. Step 5: The target mRNA is released into the cytoplasm. Step 6: The mRNA is translated into protein by the ribosome. Step 7: The protein product is degraded into antigenic peptide epitopes by the proteasome complex. Step 8: The antigenic epitopes are loaded onto MHC class I molecules in the endoplasmic reticulum. Step 9: MHC class I molecules present antigenic peptides to CD8+ T lymphocytes. Step 10: Alternatively, the protein product is secreted and then taken up by the cell, followed by a degradation process in the endosome. Step 11: The antigenic fragments are presented on the cell surface to Th cells by MHC class II molecules. Step 12: Th cells stimulate B cells to produce neutralizing antibodies against circulating pathogens. MHC, major histocompatibility complex; BCR, B cell receptor; TCR, T cell receptor; Th cell, T helper cell