Figure 2.
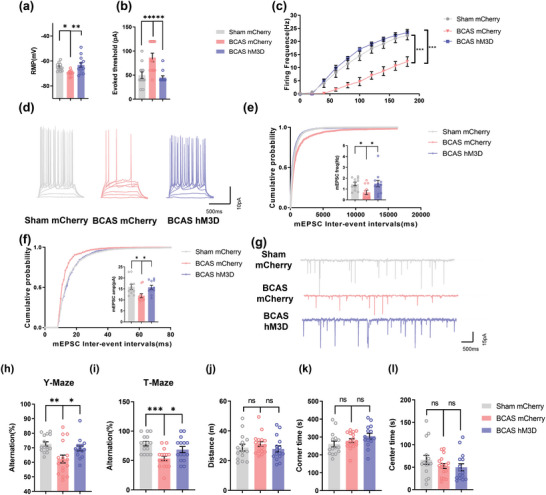
Chemogenetic stimulation ameliorates WMI‐related cognitive impairment in mice. a) The resting membrane potential (RMP), b) action potential (AP) threshold in response to current injection, and c) firing frequency of pyramidal neurons in layer 2/3 of the mPFC in the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. Recordings were acquired from 3 mice per group. n = 12 recordings per group. d) Representative APs of pyramidal neurons in layers 2/3 of the mPFC in the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. e) The frequency and f) amplitude of miniature excitatory postsynaptic currents (mEPSCs) of glutamatergic neurons in the mPFC in the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. Recordings were acquired from 3 mice per group. n = 11 recordings per group. g) Representative mEPSCs of glutamatergic neurons in the mPFC in the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. Results of h) Y‐maze and i) T‐maze tests showing the spontaneous alternation percentage of the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. n = 15–17 per group. Results of the open field test showing j) the total distance travelled and k) time spent in the corner area and l) center area in the sham mCherry, BCAS mCherry, and BCAS hM3D groups at 2 months after surgery. n = 15–17 per group. The data are presented as the mean ± SEM. p‐values were determined by 1‐way ANOVA with Tukey's post‐hoc analysis in (a), (e), (h), (i), and (k); by the Kruskal–Wallis test with Dunn's post‐hoc analysis in (b), (f), (j), and (l); and by 2‐way ANOVA with Tukey's post‐hoc analysis in (c). *p < 0.05, **p < 0.01, ***p < 0.001.
