Figure 2.
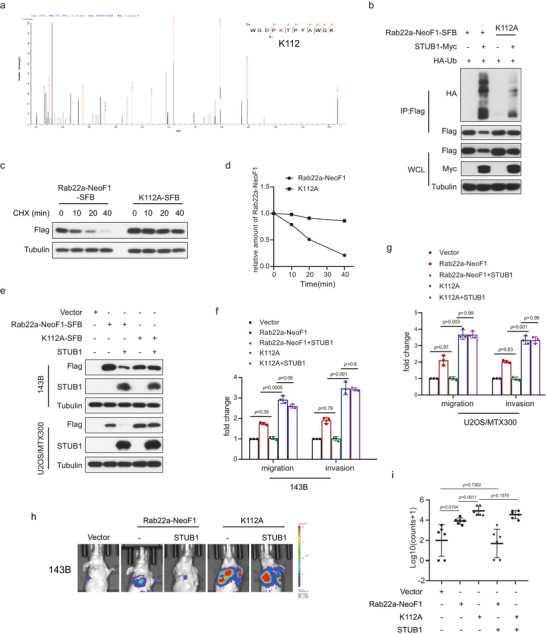
K112 is critical for the STUB1‐mediated degradation of Rab22a‐NeoF1 fusion protein. a) Lysine112 (K112) was ubiquitinated by mass spectrometry analysis of the immunoprecipitation complexes using anti‐FLAG beads from U2OS cells stably expressing Rab22a‐NeoF1‐SFB and STUB1‐Myc. b) 293T cells co‐transfected with the indicated plasmids for 48 h were lysed and immunoprecipitated using anti‐FLAG beads followed by western blot analysis. The experiments were repeated three times independently with similar results. c,d) UO2S cells stably expressing Rab2a‐NeoF1's wild type or its K112A mutant as indicated were treated with c) cycloheximide (CHX: 20 µg mL−1) for the indicated time and were subjected to western blotting analysis. d) Quantitation of the Rab22a‐NeoF1 protein levels in (c). The experiments were repeated three times independently with similar results. e) 143B cells and UO2S/MTX300 cells stably expressing Rab22a‐NeoF1's wild type or its K112A mutant were stably transfected with vector or STUB1 as indicated and then were analyzed by western blotting. The experiments were repeated three times independently with similar results. f,g) Quantification analyses of migration and invasion assays using the indicated stable cell lines shown in (e). The depicted results are the averages of at least three independent experiments. The data are presented as the mean ± SD. A two‐sided unpaired student's t‐test was performed and p values are shown. h,i) The orthotropic osteosarcoma metastasis model in vivo using the indicated stable cell lines are shown in (e) (n = 6 biologically independent animals). h) Representative images of mice. i) Quantification analyses of images.
