Table 1.
Optimization of the reaction conditionsa
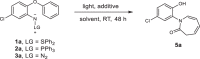 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Light source | Additive | Solvent | Yield of 5a (%)b |
| 1 | 1a | UVA | — | THF | 17 |
| 2 | 1a | blue LEDs | — | THF | 40 |
| 3 | 1a | blue LEDs | — | toluene | 14 |
| 4 | 1a | blue LEDs | — | DCE | 9 |
| 5 | 1a | blue LEDs | 10 eq. H2O | THF | 46 |
| 6 | 2a | blue LEDs | 10 eq. H2O | THF | n.d. |
| 7 | 3a | blue LEDs | 10 eq. H2O | THF | 65 |
| 8 | 3a | blue LEDs | 30 eq. H2O | THF | 45 |
| 9 | 3a | blue LEDs | — | THF/H2O = 4/1 | 28 |
| 10 | 3a | blue LEDs | 10 eq. H2O; 0.5 eq. TsOH | THF | 83 |
| 11 | 3a | blue LEDs | 10 eq. H2O; 1.0 eq. TsOH | THF | 63 |
| 12 | 3a | blue LEDs | 10 eq. H2O; 0.05 eq. TsOH | THF | 69 |
| 13 | 3a | blue LEDs | 10 eq. H2O; 0.5 eq. MsOH | THF | 69 |
| 14 | 3a | blue LEDs | 10 eq. H2O; 0.5 eq. AcOH | THF | 59 |
| 15 | 3a | blue LEDs | 10 eq. H2O; 0.5 eq. HCl | THF | 69 |
| 16 | 3a | blue LEDs | 10 eq. H2O; 0.5 eq. Zn(OTf)2 | THF | 37 |
| 17 | 3a | in the dark | 10 eq. H2O; 0.5 eq. TsOH | THF | n.d. |
Reaction conditions: 1a, 2a or 3a (0.1 mmol), additive, solvent (1.0 mL, 0.1 M), RT. a RT: room temperature; THF: tetrahydrofuran; DCE: 1,2-dichloroethane; MsOH: methanesulfonic acid; TsOH: p-toluenesulfonic acid; n.d.: not detected. b 1H NMR yield of 5a was determined by using 1,3,5-trimethoxyl benzene as the internal standard.
