Figure 7.
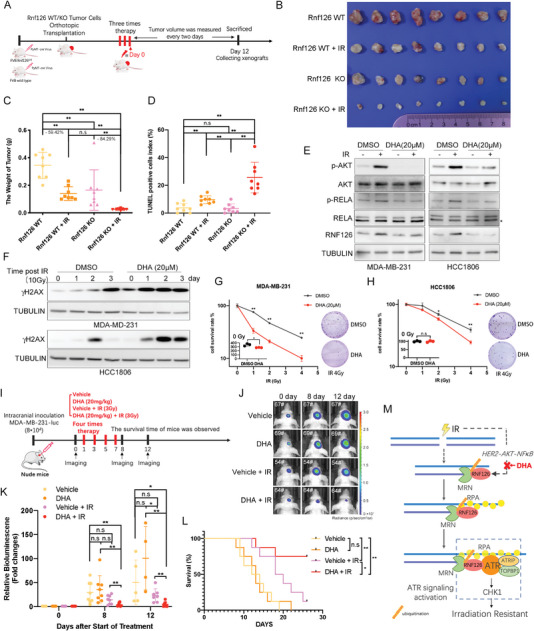
Targeting RNF126 sensitizes TNBC tumors to radiotherapy. A) Schematic diagram of radiotherapy process for PyMT‐induced Rnf126 WT/KO tumor. B–D) Rnf126 KO increased the efficacy of radiotherapy. Mice with PyMT‐induced Rnf126 WT or Rnf126 KO tumors were divided into two groups, control or radiotherapy (n = 4 per group). Twelve days post three times radiotherapy (4 Gy/time), the mice were euthanized; B) The image of tumors was shown, C) the tumors were taken to detect their weight and D) TUNEL positive ratio. Data are mean ± SD. Statistical analysis was performed using two‐tailed unpaired t‐tests. * p < 0.05 and ** p < 0.01; n.s, not significant. E) DHA inhibits RNF126 expression in both MDA‐MB‐231 and HCC1806 cells. MDA‐MB‐231 and HCC1806 cell lines were pretreated with DHA (20 µm) for 2 h before IR (10 Gy) and harvested for Western blotting analysis at 24 h point post‐IR. F) DHA pretreatment increased the accumulation of γH2AX after IR treatment in both MDA‐MB‐231 and HCC1806 cells. MDA‐MB‐231 and HCC1806 cell lines were pretreated with DHA (20 µm) for 2 h before IR (10 Gy) and harvested for Western blotting analysis at 1‐, 2‐, and 3‐day points. G,H) DHA pretreatment decreased survived clones with the increase of IR. Clonogenic survival in response to IR of MDA‐MB‐231 and HCC1806 cell lines pretreated with DHA (20 µm) for 2 h. The column represents the number of clones when only DHA (20 µm) is pretreated. Data are mean ± SD. Statistical analysis was performed using two‐tailed unpaired t‐tests. Data are representative of three independent experiments. * p < 0.05 and ** p < 0.01; n.s, not significant. I) Schematic diagram of characterization of DHA combined with radiotherapy in mice bearing intracranial MDA‐MB‐231 tumors. Cells were engineered to express luciferase. J,K) Combination of DHA and IR treatment inhibited TNBC‐derived metastatic brain tumors with the highest efficiency. Representative images of tumors in the brain imaged by J) IVIS at 0‐, 8‐, and 12‐ day points and the K) fold change of the bioluminescence signal in tumor‐bearing mice received intraperitoneal injection of the vehicle or DHA (20 mg kg−1) following with or without irradiation treatment (3 Gy, 4 times) at 0‐, 8‐, and 12‐ day points. Data in J show the mean ± SD. Statistical analysis was performed using the two‐tailed, unpaired Student's t‐test. * p < 0.05 and ** p < 0.01; n.s, not significant. L) Combination of DHA and IR treatment prolonged survival time and survival rate of the mice. Kaplan–Meier survival curves of tumor‐bearing mice receiving the indicated treatments (n = 8 per group). Statistical analysis was performed using the log‐rank test. * p < 0.05 and ** p < 0.01; n.s, not significant. M) Model of RNF126 function. We purpose IR‐induced RNF126 expression through HER2‐AKT‐NF‐κB signaling. The increasement of RNF126 induced by IR promotes the exonuclease activity of MRE11 in the case of DSBs, thus promoting ssDNA production and initiating downstream ATR‐CHK1 pathway activation, and ultimately promoting radiotherapy resistance of TNBC cells. Thus, the use of DHA to inhibit IR‐induced RNF126 expression improves the radiotherapy sensitivity of TNBC cells in the brain.
