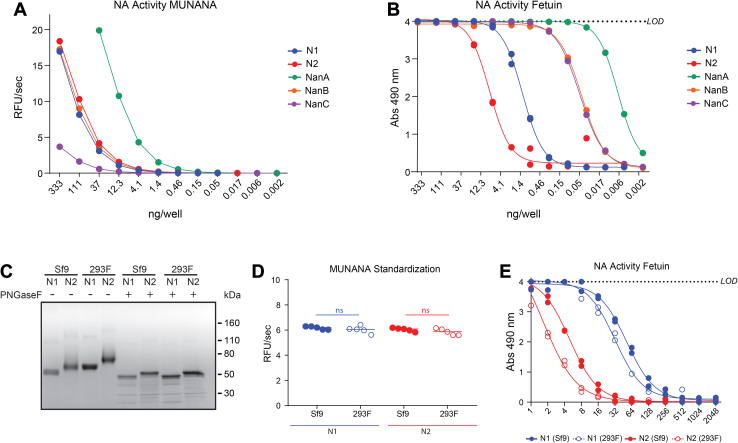
Figure 6.
Comparison of the neuraminidase activities using a biologically relevant substrate.A, specific activities of each recombinant neuraminidase were determined in the pH 6.5 ELLA buffer using MUNANA. B, graph displaying the specific activities of the recombinant proteins that were measured by an ELLA using the sialoglycans from immobilized bovine fetuin as a substrate. C, Coomassie-stained SDS-PAGE gel of recombinant N1 and N2 proteins produced in either insect (Sf9) or mammalian (293F) cells. Proteins (∼2 μg) were treated with or without PNGaseF to remove N-linked glycans prior to resolution under reducing conditions. D, recombinant N1 and N2 proteins were standardized based on activity that was measured using MUNANA. p values were generated with a two-tailed unpaired t test (95% CI). ns p > 0.05. E, the MUNANA standardized samples (from Fig. 6D) were serially diluted across a plate containing immobilized bovine fetuin, and the enzymatic activity was measured by an ELLA. MUNANA, 4-methylumbelliferyl-α-D-N-acetylneuraminide; ELLA, enzyme-linked lectin assay.