Abstract
Background
Myxoid glioneuronal tumor (MGT) is a benign glioneuronal neoplasm recently introduced in the World Health Organization (WHO) classification of the central nervous system (CNS) tumors. MGTs are typically located in the septum pellucidum, foramen of Monro or periventricular white matter of the lateral ventricle. They were previously diagnosed as dysembryoplastic neuroepithelial tumors (DNT), showing histological features almost indistinguishable from classical cortical DNT. Despite that, MGTs have been associated with a specific dinucleotide substitution at codon 385 in the platelet-derived growth factor receptor alpha (PDGFRA) gene, replacing a lysine residue with either leucine or isoleucine (p. LysK385Leu/Iso). This genetic variation has never been described in any other CNS tumor.
Materials and methods
Thirty-one consecutive tumors, previously diagnosed as DNTs at the Meyer Children's Hospital IRCCS between January 2010 and June 2021 were collected for a comprehensive study of their clinical, imaging, pathological features, and molecular profile.
Results
In six out of the thirty-one tumors we had previously diagnosed as DNTs, we identified the recurrent dinucleotide mutation in the PDGFRA. All six tumors were typically located within the periventricular white matter of the lateral ventricle and in the septum pellucidum. We then renamed these lesions as MGT, according to the latest WHO CNS classification. In all patients we observed an indolent clinical course, without recurrence.
Conclusion
MGT represent a rare but distinct group of neoplasm with a typical molecular profiling, a characteristic localization, and a relative indolent clinical course.
Keywords: Myxoid glioneuronal tumor, PDGFRA, Septum pellucidum, Dysembryoplastic neuroepithelial tumors
Introduction
In 2018, Solomon et al. reported a hotspot mutation at codon p. Lys385 of PDGFRA in a specific subgroup of dysembryoplastic neuroepithelial tumors (DNT) originated in the septum pellucidum and periventricular white matter [1]. They renamed these entity myxoid glioneuronal tumors (MGTs). This specific PDGFRA variant has not been identified as solitary genetic driver in any other central nervous system (CNS) tumor entity to date. MGT is a rare neoplasm recently introduced in the World Health Organization (WHO) classification of CNS tumors [2]. To date less than 100 cases are reported in literature. MGT have a predilection for origin in the septum pellucidum but have also been observed in the genu of the corpus callosum and periventricular white matter of the lateral ventricle. Headaches, emesis, seizures and behavioral disturbances are the most common clinical manifestations although, the presenting symptoms may vary [3].
At neuroimaging, these tumors show well-circumscribed margins, cystic features, and a discrete size (mostly 1 to 3 cm). On MRI they are mostly T1-hypointense and T2-hyperintense, with no contrast enhancement or restricted diffusion [3]; calcifications and multinodularity, which are common in cortical DNTs, are rare [4].
MGTs are mostly composed by a proliferation of oligodendrocyte-like cells embedded in a prominent myxoid stroma, and histologic features reminiscent of either DNT or rosette-forming glioneuronal tumor (RGNT) [2]. For long time these tumors have been misdiagnosed as DNT or DNT-like neoplasm of the septum pellucidum. However, MGTs lack the BRAF or FGFR1 variants or rearrangements, gene fusions, or kinase domain tandem duplication that characterize most DNTs and RGNTs [1].
MGTs are CNS WHO grade 1 tumors and are associated with a relatively indolent clinical course with favorable long-term outcome [2]. A subset of tumors can recur locally or disseminate throughout the ventricular system after subtotal resection, but they continue to be associated with indolent behavior [5]. High-grade transformation has not been described to date.
We studied six MGTs, we had previously diagnosed as DNT, with the purpose of investigating their clinical, imaging, molecular profile, and pathological features.
Patients and methods
The 31 specimens analyzed in this study were obtained from 31 patients who underwent surgery at the Neurosurgical Unit of the Meyer Children's Hospital IRCCS of Florence, Italy, between January 2010 and June 2021, and for whom tissue for both immunohistochemical staining and molecular profiling was sufficient.
Tissue specimens were routinely fixed in 10% buffered neutral formalin, paraffin embedded and stained with hematoxylin-eosin (HE) for the morphological evaluation. Pathology review of all tumors was conducted by two expert neuropathologists. Histological diagnosis was performed according to the WHO classification of central nervous system tumors [9]. Five-µm-thick sections of the most representative paraffin-embedded specimen of each case were mounted on electrostatic slides and used for immunohistochemistry. As primary antibodies we used glial fibrillary acidic protein (GFAP; clone EP672Y, Cell-Marque) Rabbit Polyclonal Anti Human OLIG2 (dilution 1:200; IBL International Hamburg, Germany), Anti-Neurofilaments (clone DA2,FNP7,RMb020.11 dilution 1:50; Thermo Fisher, Milano), Anti-NEU-N (clone A60 dilution 1:500, Invitrogen), CD34 (monoclonal, 1:25; Dako CA), BRAF (V600E, Ventana, Tucson, AZ), and Anti-Ki67 (clone Mib-1 dilution 1:100, Dako CA) on a Ventana Benchmark ULTRA immunostainer (Ventana Medical Systems, Tucson, AZ). The Ventana staining procedure included pretreatment with cell conditioner followed by incubation with antibody. The antibodies’ signal was then developed using the ultra–View Universal DAB Detection Kit. After the staining run was completed, the tissue sections were counterstained with hematoxylin.
DNA extractions from five μm-Formalin-Fixed Paraffin-Embedded (FFPE) tissues and tissue slides were performed using a manual protocol (QIAamp DNA FFPE Tissue Kit, Qiagen GmbH) and, DNA samples were quantified using the Qubit™ dsDNA HS Kit (Thermo Fisher Scientific, Wilmington, DE, USA) with a Qubit 2.0 Fluorometer. PCR primers (forward: TGCTTGTTGAAACAAAATCCTTT; reverse: CAGGCTTTCCTTGGAAGACA) for exon 8 of PDGFRA gene (NM_006206) were designed using Primer3Plus (www.primer3plus.com). PCR amplification reactions were performed in 25 μl volume reaction according to the manufacturer's instructions of HotStarTaq DNA polymerase (Qiagen GmbH), with 50 ng of DNA, 5 pmol/ul of each primers and a following cycle condition: 95°C x 15 min, 31 cycles of 93°C x 1 min, 55°C x 1 min, 72°C x 1 min; and 72°C x 10′ in the SimpliAmp™ Thermal Cycler (Applied Biosystems™, Waltham, MA, USA). Amplified PCR products were subjected to enzymatic purification using ExoSAP-ITTM (Affymetrix, Santa Clara, CA), directly sequenced on both strands with the Big Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems™, Waltham, MA, USA) according to the manufacturer's protocol in the SimpliAmp™ Thermal Cycler) and run on the 3500 Dx Series Genetic Analyzer (Applied Biosystems™; Waltham, MA, USA).
All available brain imaging studies for each patient were reviewed by two expert neuroradiologists. MRIs were performed with T13D, axial and coronal T2 weighted sequences, axial FLAIR (Fluid attenuated inversion recovery) or FLAIR 3D weighted images and T13D post intravenous contrast. CT images were acquired with the helical technique and reconstructed on the three planes with MPR algorithm. The tumors were evaluated for location and radiological features both on MRI and TC images.
Results
Microscopic examination of the 31 tumors uniformly showed low-grade proliferation of small, round monotonous oligodendrocyte-like cells (OLCs) with oval nuclei, small nucleoli and scant to moderate eosinophilic cytoplasm. A prominent myxoid stroma was present in all cases and floating neurons in an abundant mucinous matrix were seen at least focally in all tumors. Oligodendrocyte-like cells were strongly positive for Olig-2 and GFAP in all cases and all floating neurons were positive for NF and NeuN. CD34 immunoreactivity was limited to vascular endothelial cells only. BRAF V600E staining was negative in all cases. Proliferation index, evaluated with anti-Ki67 antibody, was uniformly low, ranging from <1% to 3%.
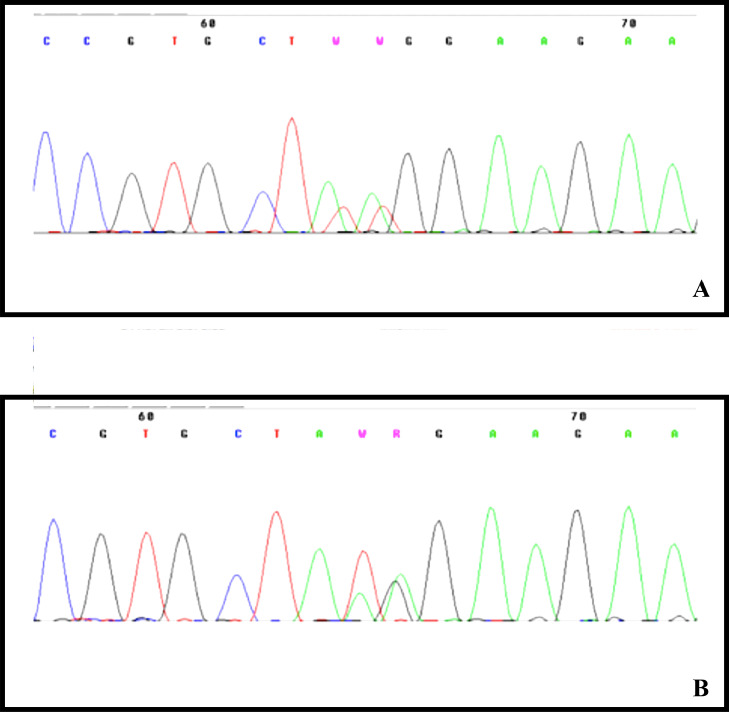
Genetic analysis of the recurrent PDGFRA p.Lys385 hotspot variants was positive in 6 out of 31 (19,35%) patients: five patients carried the c.1153_1154delAAinsTT (p.Lys385Leu) variant and one the c.1154_1155delAGinsTA (p.Lys385Ile) (Fig. 1).
Fig. 1.
Pherogram showing the PDGFRA p.Lys385 hotspot variants. Five patients carried the c.1153_1154delAAinsTT (p.Lys385Leu) variant (A) and one the c.1154_1155delAGinsTA (p.Lys385Ile) variant (B).
These six patients have a tumor located in the periventricular white matter of the lateral ventricle and in the septum pellucidum. Therefore, these six tumors were rediagnosed as MGTs.
Clinical and surgical features of the 6 MGTs specimens are listed in Table 1. Clinical and surgical features of the 25 DNTs specimens are listed in Table 2.
Table 1.
Clinical, molecular and histological features of myxoid glioneuronal tumor.
| Patient ID | Diagnosis | Molecular profile | Age at diagnosis (years) | Sex | Tumor location | Presenting symptom | Extent of resection | Adjuvant therapy | Recurrence or progression | Additional treatment after recurrence or progression | Length of follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MGT | c.1288A>T e c.1289A>T (p.Lys385Leu) | 2 | M | Periventricular white matter of lateral ventricle | Seizures | GTR | No | No | No | 10 |
| 2 | MGT | c.1289A>T e c.1290G>A (p.Lys385Ile) | 8 | M | Septum pellucidum | Seizures | GTR | No | No | No | 3 |
| 3 | MGT | c.1288A>T e c.1289A>T (p.Lys385Leu) | 8 | F | Periventricular white matter of lateral ventricle | Seizures | GTR | No | No | No | 6 |
| 4 | MGT | c.1288A>T e c.1289A>T (p.Lys385Leu) | 10 | F | Septum pellucidum | Headaches | GTR | No | No | No | 3 |
| 5 | MGT | c.1288A>T e c.1289A>T (p.Lys385Leu) | 19 | M | Periventricular white matter of lateral ventricle | Seizures | STR | No | No | No | 4 |
| 6 | MGT | c.1288A>T e c.1289A>T (p.Lys385Leu) | 14 | M | Periventricular white matter of lateral ventricle | Incidental finding | GTR | No | No | No | 1 |
MGT: Myxoid glioneuronal tumor; M: Male; F: Female; GTR: Gross total resection; STR: Sub-total resection.
Table 2.
Clinical, molecular and histological features of dysembryoplastic neuroepithelial tumor.
| Patient ID | Diagnosis | Molecular profile | Age at diagnosis (years) | Sex | Tumor location | Presenting symptom | Extent of resection | Adjuvant therapy | Recurrence or progression | Additional treatment after recurrence or progression | Length of follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DNT | WT | 15 | M | Foramen of Monro | Seizures | GTR | No | No | No | 1 |
| 2 | DNT | WT | 11 | F | T-P | Seizures | GTR | No | Yes | Surgery | 4 |
| 3 | DNT | WT | 10 | F | T | Seizures, aphasia | GTR | No | No | No | 8 |
| 4 | DNT | WT | 18 | F | T | Seizures | GTR | No | No | No | 7 |
| 5 | DNT | WT | 9 | M | T-P | Seizures | GTR | No | Yes | CHT-RT | 9 |
| 6 | DNT | WT | 34 | F | F | Seizures | GTR | No | No | No | 1 |
| 7 | DNT | WT | 17 | F | F | Seizures | GTR | No | No | No | 2 |
| 8 | DNT | WT | 21 | M | T | Seizures | STR | No | Yes | CHT | 7 |
| 9 | DNT | WT | 6 | M | F-P | Seizures | STR | No | Yes | Surgery | 6 |
| 10 | DNT | WT | 11 | M | F | Headaches, photophobia, loss of vision | GTR | No | Yes | Surgery | 7 |
| 11 | DNT | WT | 22 | F | T | Seizures | STR | No | Yes | Surgery | 6 |
| 12 | DNT | WT | 11 | F | T | Seizures | GTR | No | Yes | CHT-RT | 6 |
| 13 | DNT | WT | 16 | M | T | Seizures | STR | No | Yes | Surgery | 14 |
| 14 | DNT | WT | 9 | M | T | Headaches | GTR | No | No | No | 4 |
| 15 | DNT | WT | 8 | F | F | Seizures | GTR | No | No | No | 4 |
| 16 | DNT | WT | 17 | M | T-O | Seizures | STR | No | Yes | Surgery | 4 |
| 17 | DNT | WT | 8 | F | T | Seizures | STR | No | No | No | 3 |
| 18 | DNT | WT | 2 | M | F | Seizures | GTR | No | No | No | 2 |
| 19 | DNT | WT | 24 | F | F | Seizures | GTR | No | No | No | 2 |
| 20 | DNT | WT | 11 | F | F | Seizures | STR | No | Yes | Surgery - CHT | 3 |
| 21 | DNT | WT | 22 | M | T | Seizures | STR | No | Yes | Surgery | 1 |
| 22 | DNT | WT | 18 | F | T | Seizures | STR | No | Yes | Surgery | 2 |
| 23 | DNT | WT | 17 | M | T | Incidental finding | STR | No | Yes | Surgery | 1 |
| 24 | DNT | WT | 2 | F | T | Seizures | GTR | No | No | No | 1 |
| 25 | DNT | WT | 18 | F | T | Headaches | GTR | No | No | No | 1 |
DNT: Dysembryoplastic neuroepithelial tumor; WT: Wild type; M: Male; F: Female; T: Temporal lobe; O: Occipital lobe; F: Frontal lobe; P: Parietal lobe; GTR: Gross total resection; STR: Sub-total resection; CHT: Chemotherapy; RT: Radiotherapy.
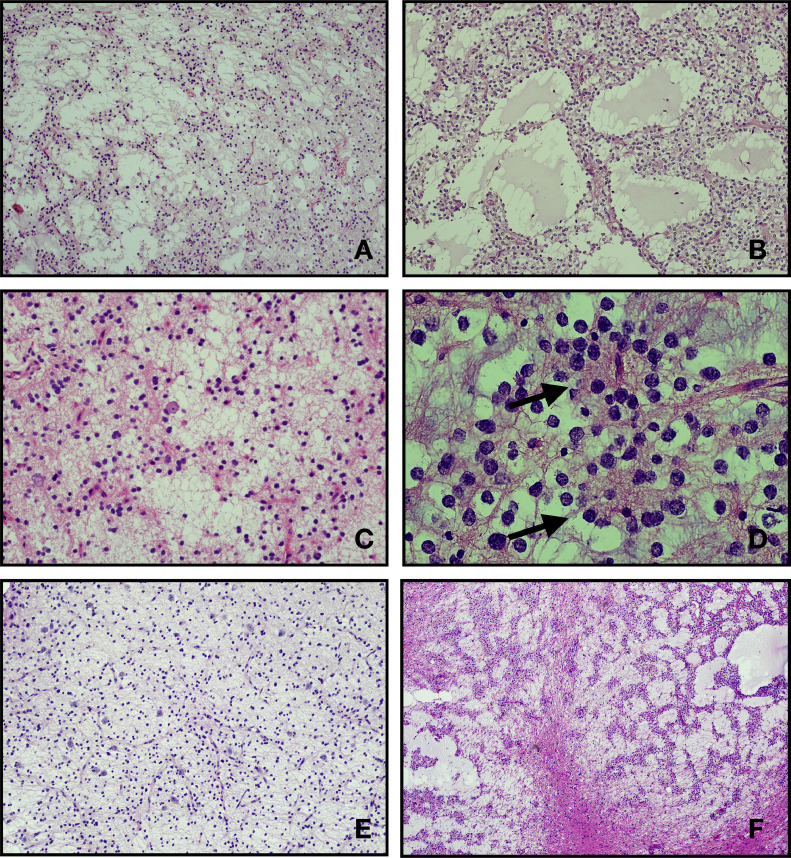
In all MGTs mitotic figures were inconspicuous (absent or less than 1 per 10 high power fields). Neither necrosis nor vascular proliferation/hyperplasia was seen in any of these tumors. Rare neurocytic rosettes were observed (MGT#4). None of the MGTs in this series showed micro calcifications. Conversely, 6 DNTs (24%; DNT#3, DNT#10, DNT#11, DNT#15, DNT#18, DNT#22) showed at least focal micro calcifications (Fig. 2).
Fig. 2.
Representative hematoxylin and eosin stained section of MGT reported in this serie. All case show a low cellularity proliferation of oligodendrocyte-like cells with monotonous round to oval nuclei, small nucleoli, and scant to moderate eosinophilic cytoplasm within a mucin-rich stroma. The tumors mostly displayed a microcytic architecture (A, B, C, F: cases MGT#1, MGT#2, MGT#3, MGT#6). In some case the tumor cells are dispose to form strands and cords (F: case MGT#6). Scattered floating neurons are seen (A, C, E: cases MGT#1, MGT#3, MGT#5). Delicate rosettes could also be rarely found (D: case MGT#4).
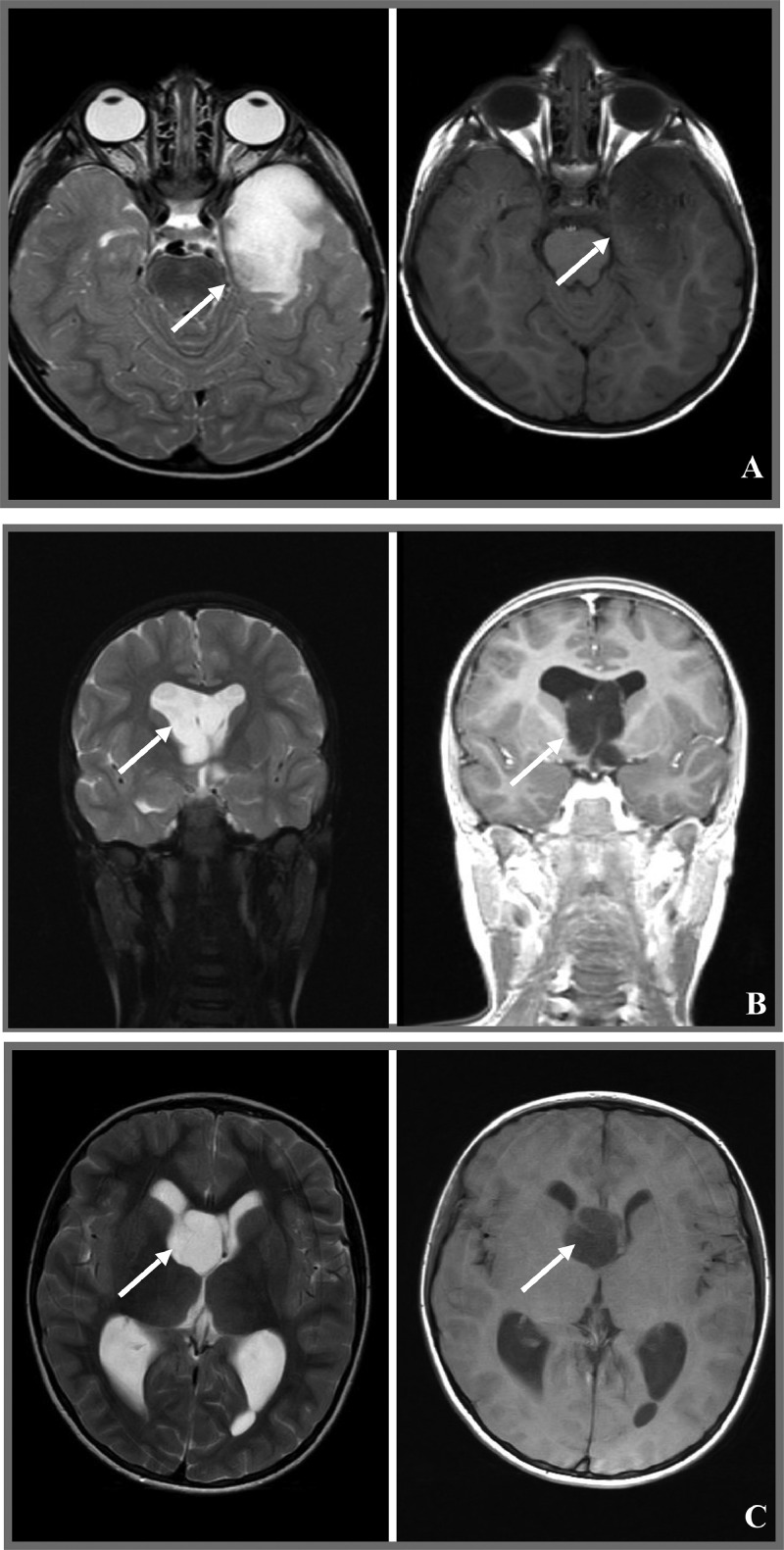
At neuroimaging, all MGT lesions in this series showed hypodensity at CT images while MR revealed weak hyperintensity in T2 and hypointensity in T1 (Fig. 3). No contrast enhancement or restricted diffusion was seen in any of the MGT patients. Calcifications and multinodularity, common features of cortical DNT, were absent in all. Obstructive hydrocephalus occurred in three out of six patients (50%) and in particular in all those whose tumor was located in the septum pellucidum. Prominent peritumoral edema was not observed. Pre-operative MRI features of the 6 MGTs and of the 25 DNTs are listed in Table 3 and Table 4 respectively.
Fig. 3.
Neuroradiologic features of MGTs reported in this series. All cases displayed a lesion with hyperintense signal in T2-weighted images and with hypointense signal in T1-weighted images, without contrast enhancement. (A: MGT#1; B: MGT#2; C: MGT#3; D: MGT#4; E: MGT#5; F: MGT#6).
Table 3.
Imaging features of myxoid glioneuronal tumor.
| Patient ID | Diagnosis | T1 signal | T2 signal | FLAIR signal | Contrast enhancement | Diffusion | Dissemination | Obstructive hydrocephalus | Parenchimal tumor involvment | Mineralization/Hemorrhage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MGT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Absent | None |
| 2 | MGT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Present, lateral ventricles | Present | None |
| 3 | MGT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Present, lateral ventricles | Absent | None |
| 4 | MGT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Present, lateral ventricles | Absent | None |
| 5 | MGT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | None |
| 6 | MGT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | NA |
MGT: Myxoid glioneuronal tumor; NA: Not available
Table 4.
Imaging features of dysembryoplastic neuroepithelial tumor.
| Patient ID | Diagnosis | T1 signal | T2 signal | FLAIR signal | Contrast enhancement | Diffusion | Dissemination | Obstructive hydrocephalus | Parenchimal tumor involvment | Mineralization/Hemorrhage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Present, lateral ventricles | Absent | None |
| 2 | DNT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | DNT | Hypointense | Hyperintense | Hyperintense | Yes | Not restricted | None | Absent | Present | Present |
| 4 | DNT | Hypointense | Hyperintense | Hyperintense | NA | Facilitated | None | Absent | Present | None |
| 5 | DNT | Hypointense | Hyperintense | Hyperintense | Yes | NA | None | Absent | Present | None |
| 6 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | None |
| 7 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | None |
| 8 | DNT | Hypointense | Hyperintense | Hyperintense | Yes | Na | None | Absent | Present | None |
| 9 | DNT | Hypointense | Hyperintense | Hyperintense | NA | NA | None | Absent | Present | None |
| 10 | DNT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | DNT | Hypointense | Hyperintense | Hyperintense | Yes | NA | None | Absent | Present | NA |
| 12 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | NA |
| 13 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | NA |
| 14 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | NA |
| 15 | DNT | Hypointense | Hypointense | Hypointense | None | Not restricted | None | Absent | Present | Present |
| 16 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | Present |
| 17 | DNT | Hypointense | Hyperintense | Hyperintense | Yes | NA | None | Absent | Present | NA |
| 18 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | None |
| 19 | DNT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 20 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | NA |
| 21 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | None |
| 22 | DNT | Hypointense | Hyperintense | Hyperintense | None | NA | None | Absent | Present | NA |
| 23 | DNT | Hypointense | Hyperintense | Hyperintense | None | Facilitated | None | Absent | Present | NA |
| 24 | DNT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 25 | DNT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
DNT: Dysembryoplastic neuroepithelial tumor; NA: Not available
Discussion
MGT, originally described and named by Solomon et al. in 2018, is a benign (WHO, grade 1) glioneuronal neoplasm [1]. To date less than 100 observations are available in the literature. These tumors, typically, arise in the periventricular region preferentially affecting the septum pellucidum, caudates nucleus, and foramina of Monro. They have an approximately equal sex distribution and predominantly occur in children and young adults, with a peak incidence in the second and third decades of life [2].
The six MGTs we describe in this series were in the typical arising sites, four in the periventricular white matter of the lateral ventricle and two in the septum pellucidum. Another tumor in our series was located in the foramen of Monro, a typical site for MGT. We were not able to demonstrate the PDGFRA recurrent variant at p.Lys385 site in this case. This processed sample presents several critical points. It was one of the first sample collected in 2011, the extraction yield was very low compared to the other samples, and the quantitative and qualitative values are not optimal. This may have affected the PCR reaction, prevented the amplification of the mutant allele and its subsequent identification by sanger sequencing. The radiological and histological characteristics of this tumor were indistinguibile from the other MGTs of this series. These features, combined with its localisation, may suggest that this tumor could be considered a MGT too.
The age of the patients ranged from 2 to 19 years, including the first available observation under the age of 3 years. The slight predominance we observed in males (4/6) is too small to be relevant.
PDGFRA activation is known to be a frequent genetic driver in many human tumor types including MGTs [1,6]. Different recurrent missense variants in PDGFRA have been identified in paediatric and adult tumors, such as the p. Cys235Tyr, p.Glu229Lys, and p.Tyr288Cys in glioblastoma and recently, the variants at codon p.Lys385 (p.Lys385Leu and p.Lys385Ile), proved to be highly specific to MGT. The high specificity of this genetic variants has been also sustained by methylation studies which suggested a distinctive methylation profile in tumors harboring an alteration at p. Lys385 residue [7]. The p. Lys385 residue falls in one of the immunoglobulin-like C2 domains in the extracellular ligand-binding portion of the receptor tyrosine kinase and the recurrent p. Lys385Leu/Ile variants are predicted to cause constitutive activation of the intracellular kinase domain [1]. We identified 5 samples (MGT#1, MGT#3, MGT#4, MGT#5, and MGT#6) carrying the dinucleotide change c.1153_1154delAAinsTT leading to the p. Lys385Leu substitution and one sample (MGT#2) carrying the c.1154_1155delAGinsTA leading to the p. Lys385Ile substitution. None of those variants were present in GnomAD (https://gnomad.broadinsitute.org/) or in HGMD (Human Gene Mutation Database, http://www.hgmd.cf.ac.uk). The c.1154_1155delAGinsTA variants was reported in the somatic COSMIC database (Catalogue of Somatic Mutations in Cancer, http://cancer.sanger.ac.uk/cosmic) associated with astrocytoma grade IV (COSM505847) [8].
Histopathologically, cortical DNTs and MGTs are indistinguishable, as they show delicate microcytic architecture, floating neurons, and homomorphous oligodendrocyte-like cells embedded in a myxoid matrix [4,2]. In contrast to classical DNTs, MGTs lack histologic aspects of multinodularity and calcifications are very rarely described. Sign of histological malignancy, such as a brisk mitotic activity, necrosis, and/or vascular proliferation are not reported. Differential diagnosis, beyond DNT, should include low-grade gliomas and low grade glioneuronal tumors such as pilocytic astrocytoma, ganglioglioma, and rosette-forming glioneuronal tumor. Therefore, a careful histological examination, a small panel of immunohistochemical markers and the demonstration of the specific PDGFRA p. Lys385 mutations at the molecular level should be considered to differentiate an MGT from its potential mimics.
Our series confirms previously reported MRI features with hypointense appearance in T1 and hyperintense signal in T2 [3]. Most of the MGTs have a cyst-like appearance and show high intensity FLAIR signal, especially in the periphery of the cyst. In line with previous reports, in none of our patients contrast enhancement or restricted diffusion were seen [9]. Calcifications, a common feature of cortical DNTs have never been observed. Obstructive hydrocephalus occurred in the two patients with tumors centered in the septum pellucidum and in the one whose tumor was centered in the periventricular white matter. The other patients did not show sign of obstructive hydrocephalus. The main differential diagnosis at neuroimaging should consider colloid cysts, ganglioglioma, pilocytic astrocytomas, sub ependymomas and central neurocytomas [10]. Colloid cysts are midline lesions, while MGTs generally are more eccentrically located [9]. Ganglioglioma and pilocytic astrocytoma are, conversely to MGTs, contrast enhancing in almost instance. Sub ependymomas are rare tumors, typically of the middle-aged to elderly patients. These are solid glial lesions with a central hyperintense signal on the FLAIR image [11]. Central neurocytomas show calcifications in about 50% of patients and a variable and heterogeneous post-gadolinium enhancement.
Clinically, MGTs have an indolent or benign clinical behavior [2]. The majority of these tumors have been successfully treated by surgery only, with no relapse during follow-up. Only a subset of tumors tend to recur locally or disseminate along the ventricular system after resection but despite this they continue to be associated with indolent behavior [5].
In our series, most MGTs (5/6) were treated with gross total surgical resection and only one patient (1/6, 17%) received a sub-total resection. None of our patients experienced a MGT recurrence at follow-up, confirming a good outcome in these patients.
In conclusion, MGT is a rare low-grade glioneuronal neoplasm are typically located in the septum pellucidum, periventricular white matter of the lateral ventricle or foramen of Monro. They exhibit a typical MRI profile, with no contrast enhancement. MGT are histologically almost indistinguishable from DNT but are unique from a molecular perspective as they are the only CNS tumor entities carrying the PDGFRA mutation as solitary genetic driver to date. Surgical resection is the treatment of choice and is associated with a good outcome even if rare disseminated or recurrent lesions have been reported.
The correct integration of the clinical, imaging, morphological and, possibly, molecular data is fundamental for a correct diagnosis.
This study was made possible in part due to The Children's Brain Tumor Network (CBTN).
CRediT authorship contribution statement
C. Caporalini: Conceptualization, Writing – original draft, Writing – review & editing. M. Scagnet: Supervision. L. Giunti: Data curation. V. Cetica: Data curation, Formal analysis, Supervision. D. Mei: Data curation, Formal analysis, Supervision. V. Conti: Data curation, Formal analysis, Supervision. S. Moscardi: Data curation. L. Macconi: Supervision. F. Giordano: Supervision. L. D'Incerti: Supervision. L. Genitori: Supervision. R. Guerrini: Writing – review & editing. A.M. Buccoliero: Conceptualization.
Declaration of Competing Interest
None.
References
- 1.Solomon D.A., Korshunov A., Sill M., Jones D.T.W., Kool M., Pfister S.M., Fan X., Bannykh S., Hu J., Danielpour M., Li R., Johnston J., Cham E., Cooney T., Sun P.P., Oberheim Bush N.A., McDermott M., Van Ziffle J., Onodera C., Grenert J.P., Bastian B.C., Villanueva-Meyer J.E., Pekmezci M., Bollen A.W., Perry A. Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a recurrent PDGFRA p.K385 mutation and DNT-like methylation profile. Acta Neuropathol. 2018;136(2):339–343. doi: 10.1007/s00401-018-1883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board . 5th ed. Vol. 6. WHO Classification of Tumours Series; WHO; Geneva, Switzerland: 2021. (Central Nervous System Tumours. Lyon (France): International Agency for Research on Cancer). [Google Scholar]
- 3.Lucas C.G., Villanueva-Meyer J.E., Whipple N., Oberheim Bush N.A., Cooney T., Chang S., McDermott M., Berger M., Cham E., Sun P.P., Putnam A., Zhou H., Bollo R., Cheshier S., Poppe M.M., Fung K.M., Sung S., Glenn C., Fan X., Bannykh S., Hu J., Danielpour M., Li R., Alva E., Johnston J., Van Ziffle J., Onodera C., Devine P., Grenert J.P., Lee J.C., Pekmezci M., Tihan T., Bollen A.W., Perry A. Solomon DAMyxoid glioneuronal tumor, PDGFRA p.K385-mutant: clinical, radiologic, and histopathologic features. Brain Pathol. 2020;30(3):479–494. doi: 10.1111/bpa.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thom M., Toma A., An S., Martinian L., Hadjivassiliou G., Ratilal B., Dean A., McEvoy A., Sisodiya S.M., Brandner S. One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetics, and clinical correlations and a review of the literature. J. Neuropathol. Exp. Neurol. 2011;70(10):859–878. doi: 10.1097/NEN.0b013e3182302475. [DOI] [PubMed] [Google Scholar]
- 5.Kleinschmidt-DeMasters B.K., Chiang J., Donson A.M., Borges T., Gilani A. Myxoid glioneuronal tumor, PDGFRA p.K385L-mutant, arising in midbrain tectum with multifocal CSF dissemination. Brain Pathol. 2022;32(1):e13008. doi: 10.1111/bpa.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip C.K.M., Ng P.K.S., Jeong K.J., Shao S.H., Ju Z., Leonard P.G., Hua X., Vellano C.P., Woessner R., Sahni N., Scott K.L., Mills G.B. Neomorphic PDGFRA extracellular domain driver mutations are resistant to PDGFRA targeted therapies. Nat. Commun. 2018;9(1):4583. doi: 10.1038/s41467-018-06949-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang J.C.H., Harreld J.H., Tanaka R., Li X., Wen J., Zhang C., Boué D.R., Rauch T.M., Boyd J.T., Chen J., Corbo J.C., Bouldin T.W., Elton S.W., Liu L.L., Schofield D., Lee S.C., Bouffard J.P., Georgescu M.M., Dossani R.H., Aguiar M.A., Sances R.A., Saad A.G., Boop F.A., Qaddoumi I., Ellison D.W. Septal dysembryoplastic neuroepithelial tumor: a comprehensive clinical, imaging, histopathologic, and molecular analysis. Neuro. Oncol. 2019;21(6):800–808. doi: 10.1093/neuonc/noz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartzentruber J., et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 9.Zamora C., Castillo M. From dysembryoplastic neuroepithelial tumors to myxoid glioneuronal tumors, a new entity. AJNR Am. J. Neuroradiol. 2021;42(11):E77–E78. doi: 10.3174/ajnr.A7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baisden B.L., Brat D.J., Melhem E.R., Rosenblum M.K., King A.P., Burger PC. Dysembryoplastic neuroepithelial tumor-like neoplasm of the septum pellucidum: a lesion often misdiagnosed as glioma: report of 10 cases. Am. J. Surg. Pathol. 2001;25(4):494–499. doi: 10.1097/00000478-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Narvaez E.O., Inada B.S.Y., de Almeida P., Freitas L.F., Soldatelli M.D., Costa D.M.C., Marussi V.H.R., Campos C.S., Vitorino Araujo J.L., Carrete Junior H., do Amaral L.L.F. Myxoid glioneuronal tumor - report of three cases of a new tumor in a typical location and review of literature. BJR Case Rep. 2021;7(4) doi: 10.1259/bjrcr.20200139. [DOI] [PMC free article] [PubMed] [Google Scholar]