Figure 3.
Deletion of CD81-LEL in lung epithelial cells reduces internalization of bacilli and intracellular bacterial loads
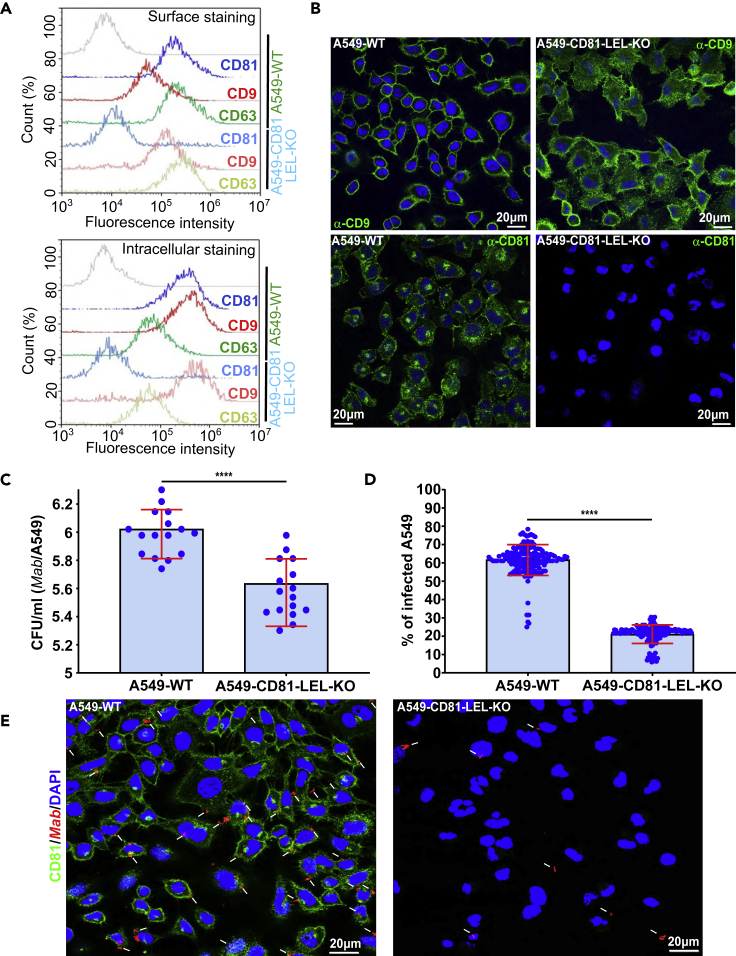
(A) A549-WT or A549-CD81-LEL-KO cells were harvested and fixed in 2% paraformaldehyde before washing and incubated with indicated primary antibodies, followed by staining with Alexa Fluor 647-coupled secondary antibodies in non-permeabilizing (surface staining) or permeabilizing (intracellular staining) buffer. Cells were then washed and processed through a Novocyte ACEA cytometer. Surface staining (upper inset) and intracellular staining (lower inset) are represented as histogram overlays for each tetraspanin expression in both cell types (CD81 in blue; CD9 in red; CD63 in green). The staining control was done with incubation of secondary antibodies alone (gray curves).
(B) Localization of tetraspanins CD9 and CD81 (green staining) in A549_WT cells and in A549-CD81-LEL-KO cells. Because these antibodies are directed against the LEL, no CD81 signal is detected in A549-CD81-LEL-KO cells.
(C) Impact of CD81-LEL disruption in A549 cells on the internalization of Mab. CFUs were determined at 3 h post-infection, as described previously. Data are the means ± SD values for four independent experiments performed in quadruplicate (n = 16). One-tailed Tukey’s test: ns, non-significant > 0.05; ∗∗∗∗p < 0.0001.
(D) Deletion of CD81-LEL significantly diminishes the uptake of Mab and the percentage of infected A549 cells. Quantification of the percentage of host cells containing bacilli was performed as in Figure 1C. Data are mean values ±SD for eight independent experiments (n = 160 fields). One-tailed Tukey’s test: ns, non-significant > 0.05, ∗∗∗∗p < 0.0001.
(E) Immunofluorescence confocal microscopy fields showing A549 WT, or A549-CD81-LEL-KO cells infected with red-fluorescent mycobacteria (white arrows). The cell surface is stained using anti-CD81 antibodies (green) whereas the nuclei are stained with DAPI (blue).