Extended Data Fig. 5: Conformational changes in structures containing the METTL1-WDR4-tRNA complex.
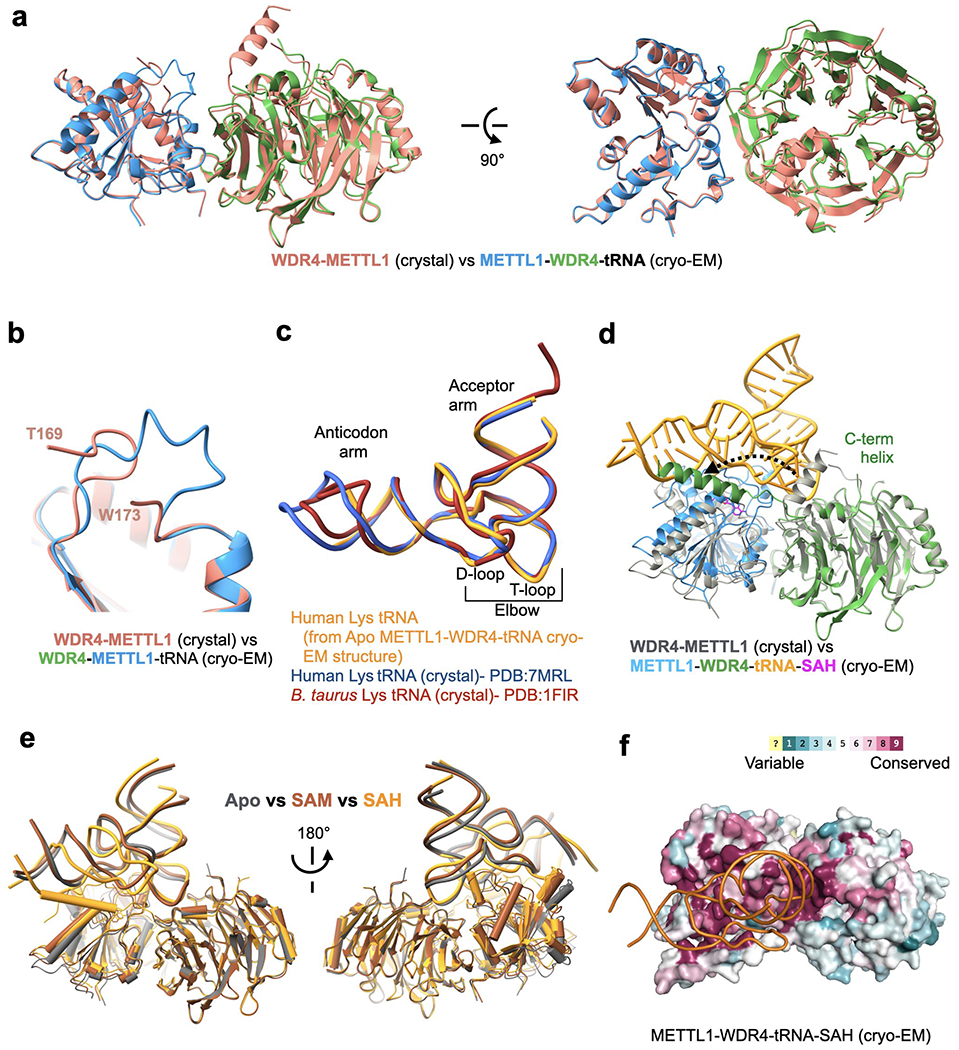
a, Superimposition of the METTL1-WDR4 crystal structure on the METTL1-WDR4-tRNA cryo-EM structure, aligned by METTL1. b, Flexible loop of METTL1 (161-175, catalytic loop) becomes more ordered with RNA. c, Superimposition of different tRNALys structures. All three have the same sequence except the tips of the anticodon arm and the acceptor arm. The B. Taurus structure (PDB:1FIR) was with a fully modified tRNA and the other two structures were obtained for unmodified RNA after in vitro transcription. d, Superimposition of the METTL1-WDR4 crystal structure (gray) onto the SAH-bound quaternary complex cryo-EM structure (multiple colors), aligned by WDR4. Movement of the WDR4 C-terminal helix upon binding RNA is shown with a dashed arrow. e, Superimposition of all three cryo-EM structures (colored by state) presented in this study, aligned by WDR4. f. Surface representation of the SAH-bound cryo-EM structure colored by evolutionary sequence conservation (Consurf server). The orientation is identical to Fig. 3a and 3b.
