Fig. 3: Structural recognition of tRNA shape by METTL1-WDR4.
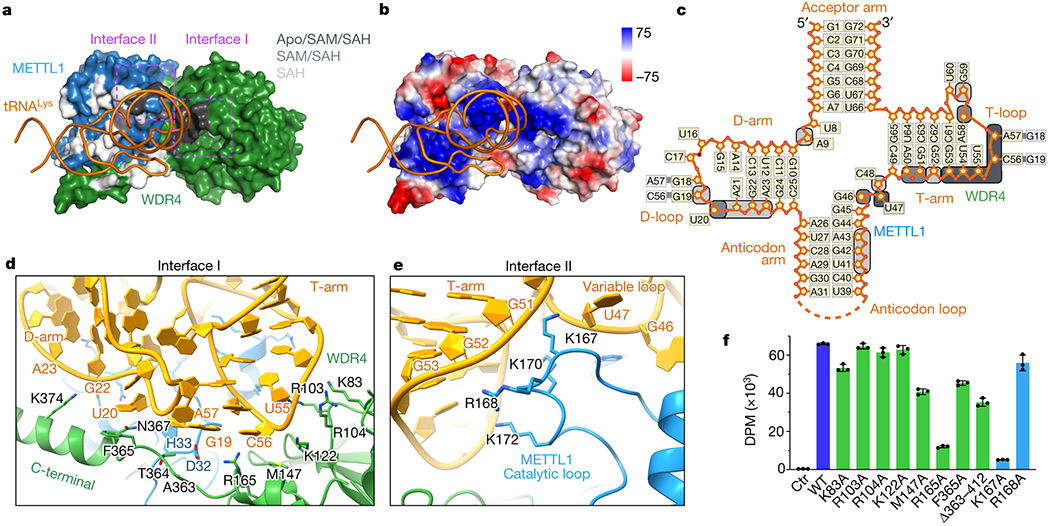
a, Surface representation of the METTL1-WDR4-tRNA-SAH structure, with only tRNA in ribbon representation. Residues within 4 Å of RNA are colored by conservation of the protein-RNA contact in the three states: black (Apo/SAM/SAH), grey (SAM/SAH), or white (SAH only). Two interface areas with conserved contacts in all three states are marked with dotted rectangles in magenta. b, Vacuum electrostatics surface representation of the protein complex in the same orientation as (a). c, Secondary structure diagram of tRNALys. Nucleotides within 4 Å of protein are indicated with rounded squares and colored using the same color key as in (a). d-e, Close-up views of METTL1-WDR4-tRNA-SAH where the residues within 4 Å of RNA are shown with side chain sticks for the interfaces marked in (a). f, In vitro methylation activity of METTL1-WDR4 with point mutations in WDR4 (green) or METTL1 (blue), shown as mean ± SD from 3 replicates.
