Fig. 6: Mechanistic model for tRNA m7G46 methylation by METTL1-WDR4.
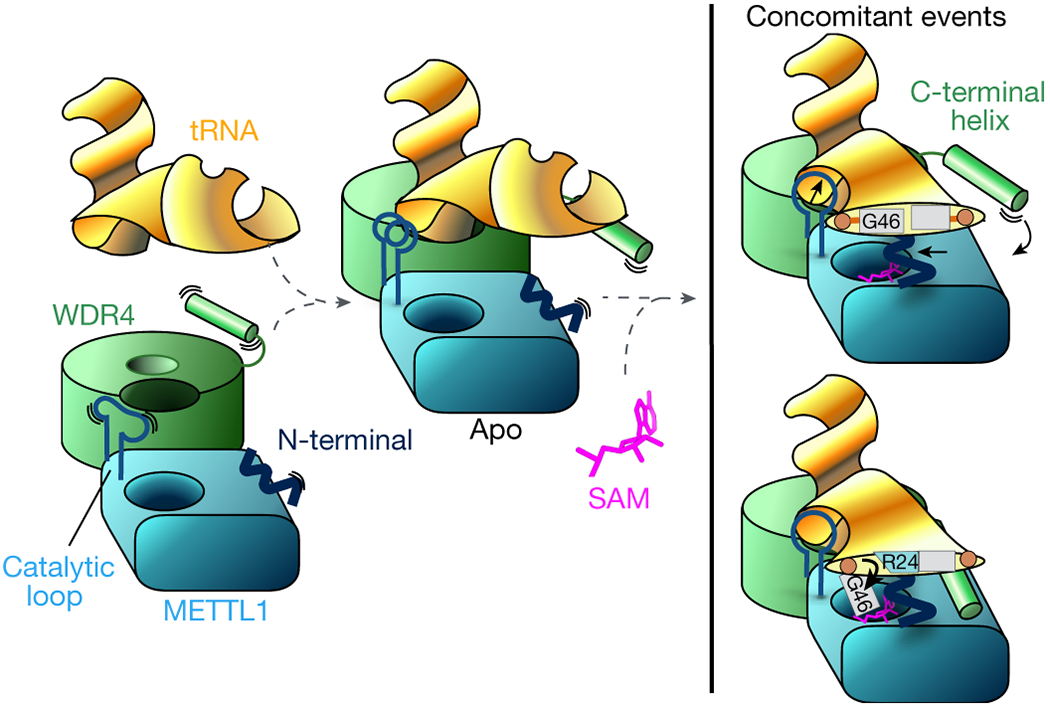
The stable heterodimeric METTL1-WDR4 protein complex provides a docking site for the tRNA Elbow. The protein-RNA docking can occur without cofactor, and METTL1 can bind SAM without tRNA. In the apo state without SAM or SAH, the catalytic loop becomes more ordered with bound RNA. WDR4 C-terminal helix moves close to RNA but remains flexible and METTL1 N-term is also disordered. When both tRNA and SAM are bound, METTL1 shifts even closer to the tRNA—the catalytic loop protrudes toward the tRNA, METTL1 N-term becomes ordered sandwiched between RNA and SAM, and WDR4 C-term attaches to the METTL1 N-term to stabilize the bound RNA together. The same N-term also supports the twisting of the tRNA to release G46 by replacing the stacking interactions with R24.
