Extended Data Fig. 1: METTL1-WDR4 protein purification and tRNA complex reconstitution.
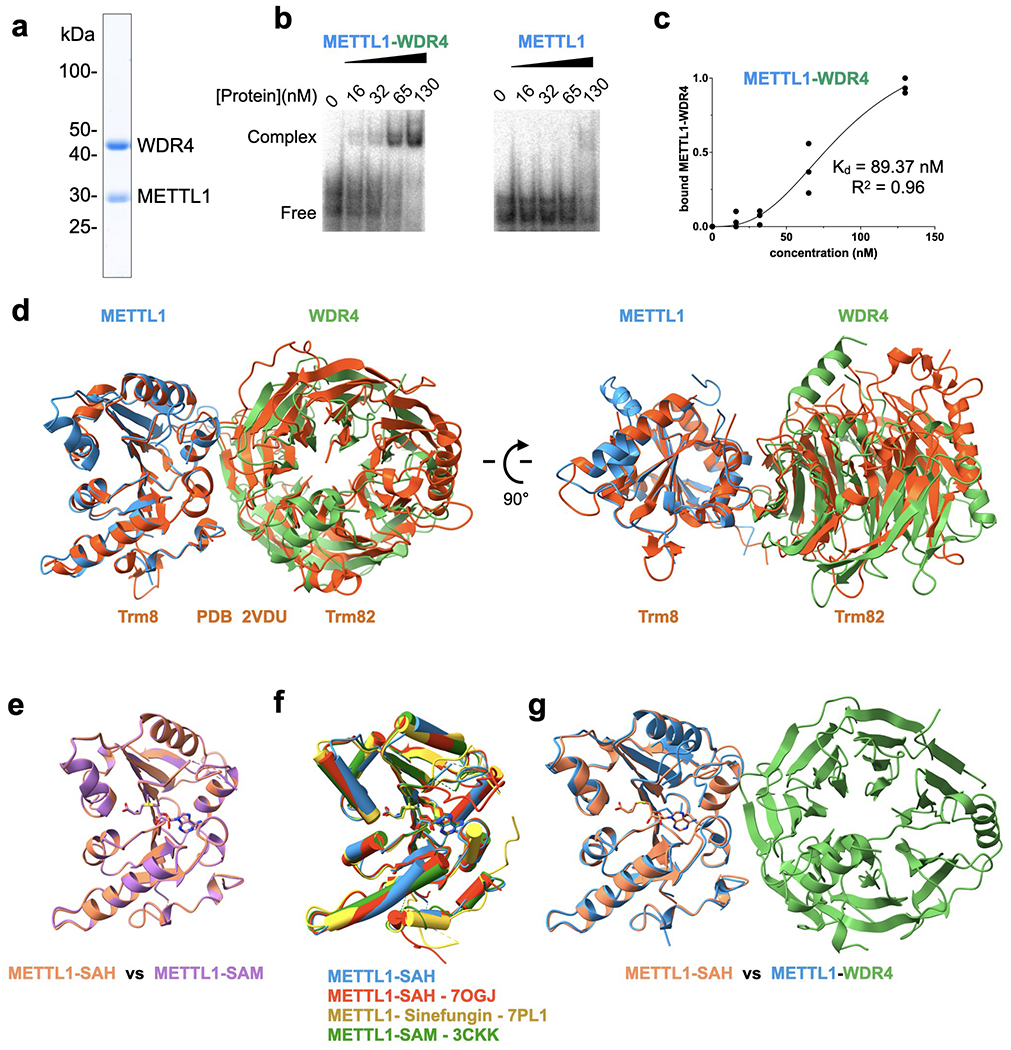
a, SDS-PAGE of purified full-length wild-type METTL1-WDR4 complex. A representative gel among 3 replicates is shown. b, EMSA to measure the affinity for tRNALys. For each gel, protein concentrations are 0, 16, 32, 65, and 130 nM, left to right. Representative gel among 3 replicates is shown. c, Quantification of EMSA for METTL1-WDR4 from 3 replicate experiments. d, Superimposition of yeast Trm8-Trm82 (PDB 2VDU, orange) onto the crystal structure of human METTL1-WDR4. The complex structures were superimposed by aligning METTL1 with Trm8. e-g, Superimposition of METTL1 structures as indicated. The structures missing PDB codes are from this study.
