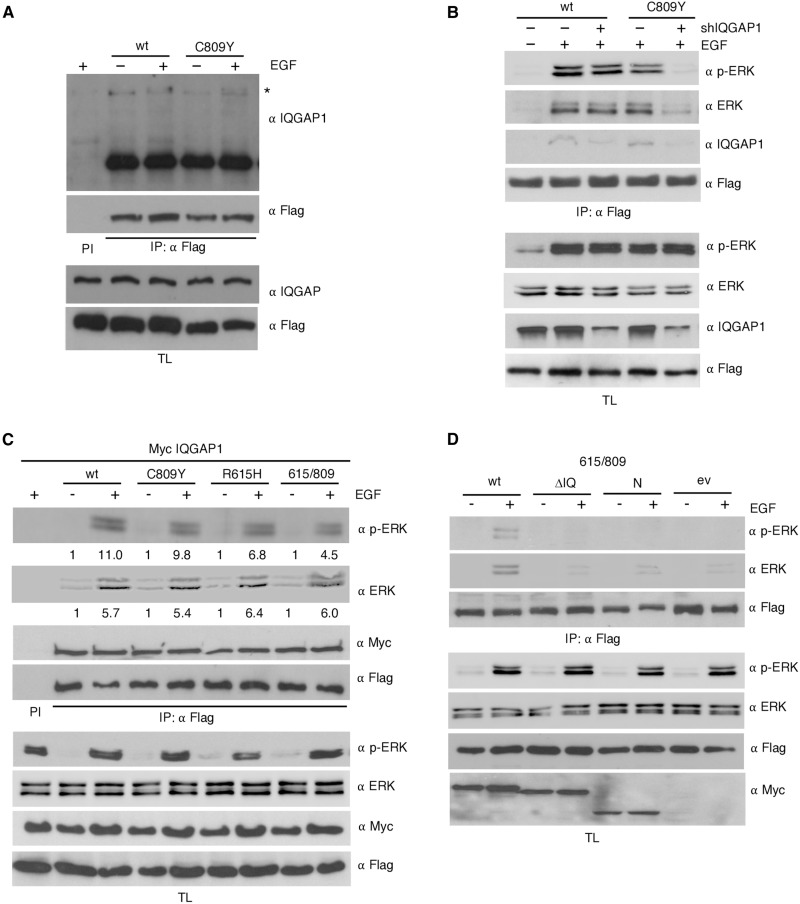
Fig. 4. IQGAP1 participates in KSR1 trans-phosphorylation.
(A) IQGAP1 interacts with KSR1 C809Y. Coimmunoprecipitation assay from HEK293T cells cotransfected with the indicated Flag-tagged KSR1 constructs (1.5 μg each), upon EGF stimulation (50 ng/ml, 5 min) where indicated (+), after 18-hour starvation (−). (B) Effects of IQGAP1 depletion on phosphorylated ERK binding to KSR1 C809Y. Coimmunoprecipitation assay in KSR1−/− MEFs expressing the indicated Flag-tagged KSR1 constructs and transfected with shRNA against IQGAP1 where indicated (+) (1 μg), following EGF stimulation (+). (C) IQGAP1 overexpression facilitates KSR1 incorporation of phosphorylated ERK. Coimmunoprecipitation from HEK293T cells transfected with the indicated Flag-tagged KSR1 constructs plus Myc-tagged IQGAP1, in starved cells (−) or upon EGF stimulation where indicated (+). Figures show signal intensity relative to the levels in starved cells. (D) KSR1 incorporation of phosphorylated ERK mediated by IQGAP1 KSR-binding and MEK-binding mutants. Coimmunoprecipitation from HEK293T cells transfected with both Flag-tagged KSR1 615/809 and the indicated Myc-tagged IQGAP1 mutants (1 μg each), in starved cells (−) or upon EGF stimulation as shown (+); ev, empty vector. In all cases, immunoprecipitations were performed with a specific antibody (IP) or with preimmune serum (PI). All the results shown are representative of three to five independent experiments (see also fig. S3).