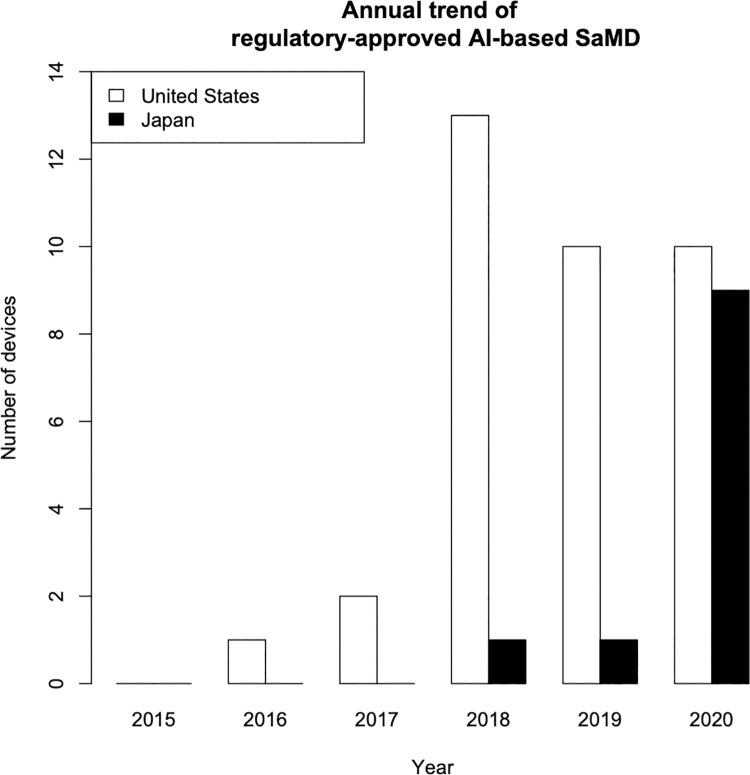
Fig 4. Comparison of the approval status of machine learning/deep learning-based software as a medical device (SaMD) between Japan and the United States.
The figure illustrates a comparison between the current approval number/year in Japan and the US. The status of the US refers to the study conducted by Benjamens et al. [14] The state of 2020 in the US is shown by the simulated number from the study as it terminated in February 2020 unlike our study that terminated in October 2020. Note that the study conducted by Benjamens et al. evaluated the number of regulatory-approved AI/ML-based SaMD, whereas we evaluated regulatory-approved ML/DL-based SaMD. This is because the term “artificial intelligence” is too broad in meaning to represent the recent fourth industrial revolution. Even with discrepancy in terminology between the two studies, the figure illustrates the trend of the rapidly increasing Japanese approval number and the trend that follows the recent status of the US.