FIGURE 4.
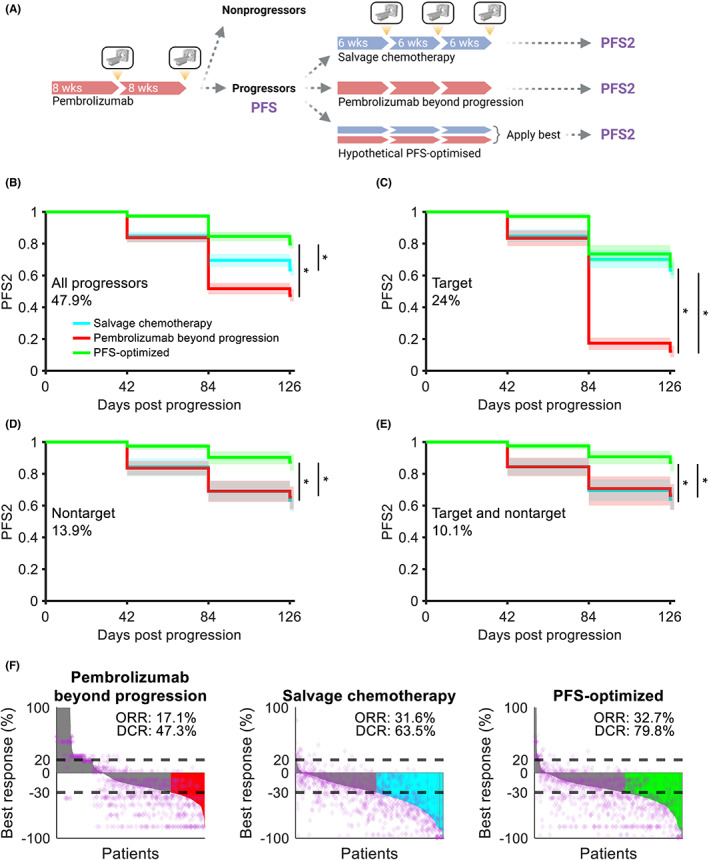
Pembrolizumab beyond progression can prolong PFS in nontarget progressors. (a) Schematic of a virtual clinical trial comparing pembrolizumab beyond progression versus salvage chemotherapy or a hypothetical PFS‐optimized regimen. Treatment with pembrolizumab was simulated for up to 16 weeks with radiographic assessment for response every 8 weeks. Progressors were cloned in silico and assigned to receive treatment for 18 weeks with radiographic assessment for response every 6 weeks. (b) PFS2 in patients receiving pembrolizumab beyond progression, salvage chemotherapy, or a hypothetical PFS‐optimized treatment (see Methods). Data are pooled across 100 trial replicates, with solid lines representing medians and shaded regions representing the 5th and 95th percentiles. (c) Same as (b) for patients with target progression without nontarget progression. (d) Same as (b) for patients with nontarget progression without target progression. (e) Same as (b) for patients with simultaneous target and nontarget progression at radiographic assessment. (f) Best response, ORR, and DCRs in patients after treatment. See the Methods for calculation. Data are pooled across 100 replicates. Horizontal dashed lines indicate thresholds for progressive disease and partial response, purple diamonds represent individual lesions from a representative replicate, and colored bars indicate patients with responses and no nontarget progression. Gray bars indicate patients with no response as well as patients with target lesion responses but simultaneous nontarget progression. *p < 0.0005 (log‐rank test). DCR, disease control rate; ORR, objective response rate; PFS, progression‐free survival; PFS2, progression‐free survival under subsequent therapy
