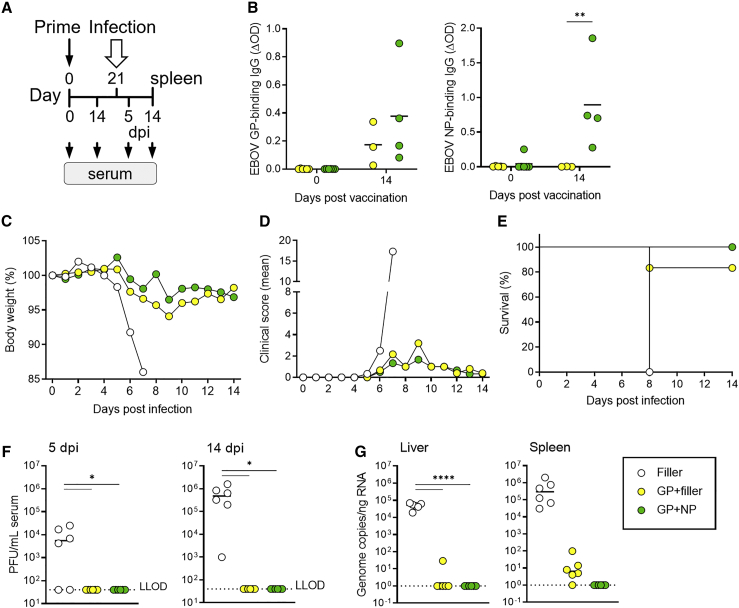
Figure 5.
Protection against lethal EBOV infection induced by single immunization with GP or GP + NP saRNA
C57BL/6J IFNAR−/− mice (six per group) were injected on day 0 only. The vaccine contained 5 μg GP saRNA alone + 2.5 μg filler (GP + filler) or combined with 2.5 μg NP saRNA (GP + NP). The filler saRNA was formulated into LNPs in a total dose of 7.5 μg to keep the absolute RNA amount constant in all samples. Serum samples were collected before immunization (day 0) and on day 14 after immunization. Mice were challenged i.p. with 1,000 PFU of EBOV on day 21, and survival was recorded for 14 days (six per group). (A) Schematical overview of the experimental setting. (B) Seroconversion per group over time (three or four mice per group). ELISA for EBOV GP and EBOV NP was performed in serum samples (1:100 dilution) from the indicated days, as described in Figure 2. (C) Body weight curves for the different groups after infection. (D) Clinical scores. Mice that developed severe clinical signs of infection and/or exceeded 15% of body weight loss were euthanized. (E) Survival over time. Survival over time is shown in the graph on the right. (F) Infectious EBOV particles were quantified by plaque titration in serum samples from days 5 and 14 after infection or at the day of the sacrifice. (G) EBOV genome copies in liver and spleen samples from day 14 p.i. or from the day of the sacrifice, by EBOV GP-specific qRT-PCR. Symbols indicate individual mouse values, with group mean values indicated by horizontal lines for all graphs, except for (C–E), where symbols represent group average values of six mice per group. Asterisks indicate statistical significance as detailed by bars between relevant groups: ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗∗p < 0.0001.