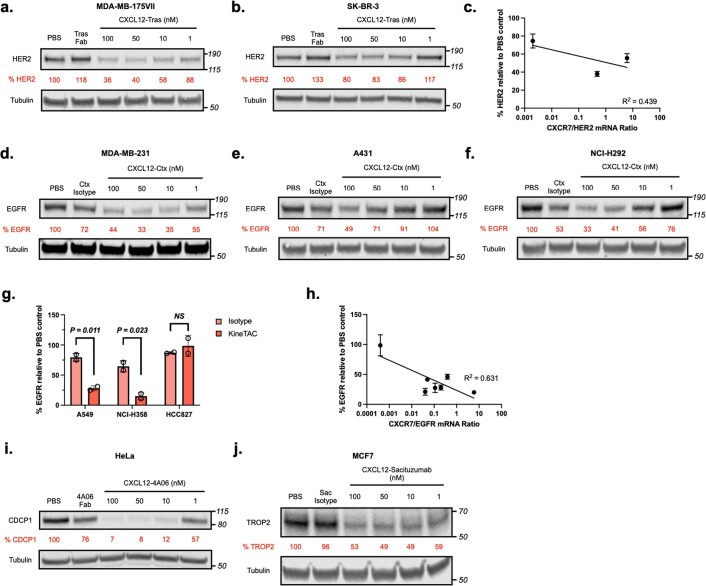
Extended Data Fig. 2. KineTACs mediate degradation of additional therapeutically relevant cell surface proteins.
Dose escalation showing HER2 degradation in a, MDA-MB-175VII and b, SK-BR-3 cells following treatment with CXCL12-Tras or 100 nM trastuzumab Fab. c, Trend of HER2 levels to CXCR7/HER2 transcript level ratio as calculated by densitometry after treatment with 100 nM CXCL12-Tras for 24 hrs in MCF7, MDA-MB-175VII, and SK-BR-3 cells. N = 2 biologically independent experiments. Dose escalation showing EGFR degradation in d, MDA-MB-231, e, A431, and f, NCI-H292 cells following treatment with CXCL12-Ctx or 100 nM Cetuximab isotype. g, EGFR levels after treatment with 100 nM CXCL12-Ctx were significantly reduced compared to Ctx isotype control for 24 hrs in non-small cell lung cancer cell lines A549 (P = 0.011) and NCI-H358 (P = 0.023) but were unchanged in HCC827 (P = 0.4559) where EGFR is substantially overexpressed. N = 2 biologically independent experiments. h, Trend of EGFR levels to CXCR7/EGFR transcript level ratio as calculated by densitometry after treatment with 100 nM CXCL12-Ctx for 24 hrs in HeLa, A431, NCI-H292, MDA-MB-231, A549, NCI-H358, and HCC827 cells. N = 3 biologically independent experiments for HeLa and N = 2 biologically independent experiments for remaining cell lines. i, Dose escalation showing CDCP1 degradation in HeLa cells following treatment with CXCL12-4A06 or 100 nM 4A06 Fab. j, Dose escalation showing TROP2 degradation in MCF7 cells following treatment with CXCL12-Sacituzumab or sacituzumab isotype. Densitometry was used to calculate protein levels and normalized to PBS control. Data are represented as mean values and error bars represent the standard deviation of biological replicates. P-values were determined by unpaired two-tailed t-tests.