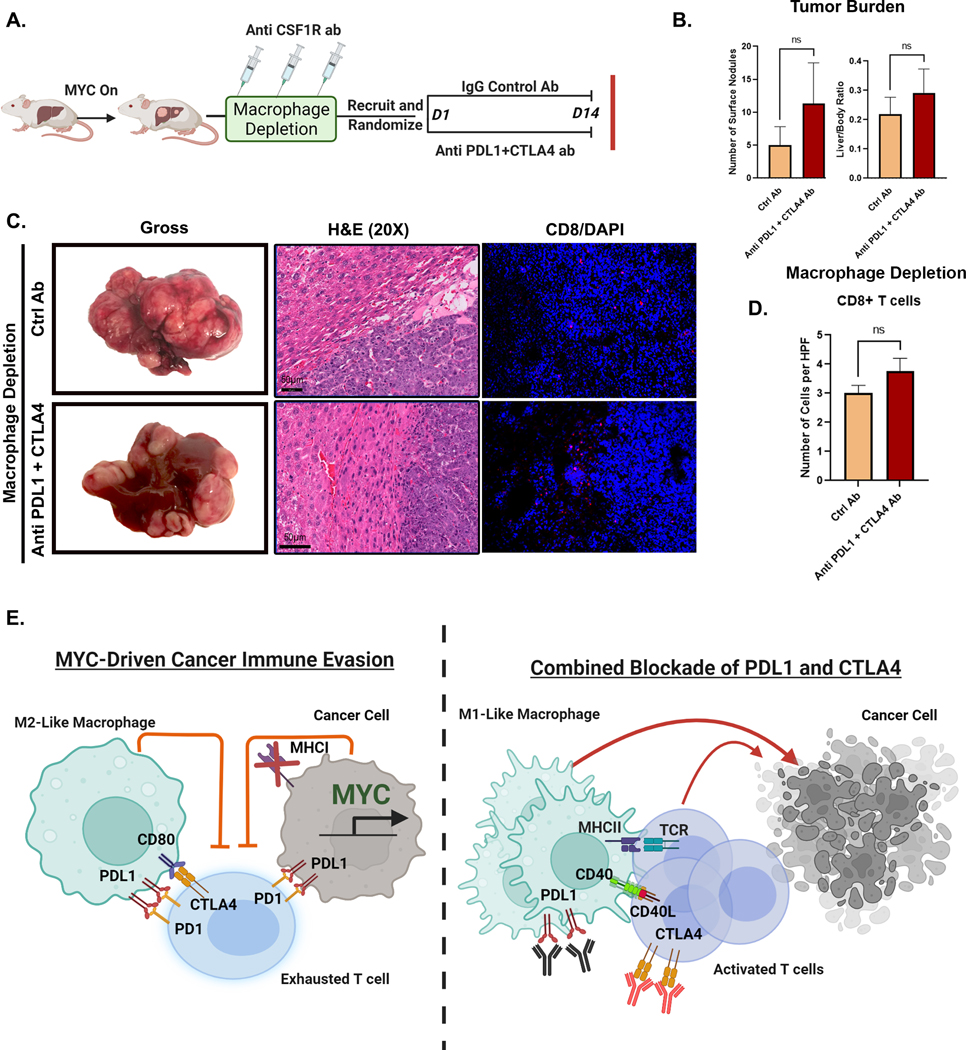
Figure 7. Macrophages are Essential for the anti-tumor efficacy of Combined Immune Checkpoint Therapy in MYC-HCC.
A. Experimental scheme of macrophage depletion in MYC-HCC followed by treatment with either IgG control (n=4) or dual PDL1 and CTLA4 antibodies (n=3) (created using Biorender.com).
B. Quantification of tumor burden at end-of-treatment with either IgG control (n=4) or dual PDL1 and CTLA4 antibodies (n=3) in macrophage-depleted MYC-HCC mice.
C. End-of-treatment gross appearance, histology and immunofluorescence for CD8 T cells in representative macrophage-depleted MYC-HCC-bearing mice treated with IgG control (n=4) or dual PDL1 and CTLA4 antibodies (n=3).
D. Quantitation of CD8T cell infiltration in macrophage-depleted MYC-HCC-bearing mice treated with IgG control (n=4) or dual PDL1 and CTLA4 antibodies (n=3).
E. Figurative representation of the mechanism of efficacy of combined PDL1 and CTLA4 therapy in MYC-HCC. MYC-driven cancers are immune evasive with MHCI repression and PDL1 overexpression on cancer cells and macrophages which in turn lead to T cell exhaustion. Treatment with CTLA4 and PDL1 inhibitors leads to repolarization of macrophages to the M1-like phenotype with increased expression of CD40 and MHCII, which leads to enhanced antigen presentation and robust T cell activation resulting in delayed tumor progression (created using Biorender.com).