Abstract
Aim
The recent introduction of long axial field-of-view (LAFOV) PET/CT scanners has yielded very promising results regarding image quality and sensitivity in oncological patients. We, herein, aim to determine an appropriate acquisition time range for the new long axial field of view Biograph Vision Quadra PET/CT (Siemens Healthcare) using low dose [18F]FDG activity in a group of melanoma patients.
Methodology
Forty-nine melanoma patients were enrolled in the study. All patients underwent total body PET/CT from the top of the head through the feet in two bed positions (field-of-view 106 cm) after i.v. injection of 2.0 MBq/kg [18F]FDG. The PET images of the first bed position (head to upper thigh; PET-10) were reconstructed and further split into 8-min (PET-8), 6-min (PET-6), 5-min (PET-5), 4-min (PET-4), and 2-min (PET-2) duration groups. Comparisons were performed between the different reconstructed scan times with regard to the visual evaluation of the PET/CT scans using the PET-10 images as reference and by calculating the 95%-CI for the differences between different time acquisitions. Moreover, objective evaluation of PET/CT image quality was performed based on SUV calculations of tumor lesions and background, leading to calculation of liver signal-to-noise ratio (SNR), and tumor-to-background ratio (TBR).
Results
A total of 60 scans were evaluated. Concerning visual analysis, 49/60 (81.7%) PET-10 scans were pathological, while the respective frequencies were 49/60 (81.7%) for PET-8 (95%-CI: − 0.0602–0.0602), 49/60 (81.7%) for PET-6 (95%-CI: − 0.0602–0.0602), 48/60 (80%) for PET-5 (95%-CI: − 0.0445–0.0886), 46/60 (76.7%) for PET-4 (95%-CI: − 0.0132–0.1370), and 45/60 (75%) for PET-2 (95%-CI: 0.0025–0.1593). In 18 PET-10 scans, the extent of metastatic involvement was very large, rendering the accurate calculation of [18F]FDG-avid tumor lesions very complicated. In the remaining 42 PET-10 scans, for which the exact calculation of tumor lesions was feasible, a total of 119 tumor lesions were counted, and the respective lesion detection rates for shorter acquisitions were as follows: 97.5% (116/119) for PET-8 (95%-CI: 0–1), 95.0% (113/119) for PET-6 (95%-CI: 0–1), 89.9% (107/119) for PET-5 (95%-CI: 0–2), 83.2% (99/119) for PET-4 (95%-CI: 1–2), and 73.9% (88/119) for PET-2 (95%-CI: 2–4). With regard to objective image quality evaluations, as a general trend, the reduction of acquisition time was associated with a decrease of liver SNR and a decrease of TBR, although in lesion-based analysis the change in TBR and tumor SUVmean values was non-significant up to 6 and 5 min acquisitions, respectively.
Conclusions
In melanoma, low-dose LAFOV PET/CT imaging is feasible and can reduce the total scan time from head to upper thigh up to 5 min providing comparable diagnostic data to standard lengths of acquisition. This may have significant implications for the diagnostic work-up of patients with melanoma, given the need for true whole-body imaging in this type of cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-022-06070-7.
Keywords: Melanoma, LAFOV PET/CT, [18F]FDG, Total body, Whole-body, SUV
Introduction
Positron emission tomography (PET) integrated with computed tomography (CT) is a whole-body imaging technique combining functional and morphological information in one procedure. PET/CT, mainly with the radiotracer 2-deoxy-2-[fluorine-18] fluoro-D-glucose ([18F]FDG), the workhorse of molecular imaging, is nowadays a standard imaging method applied in the clinical routine of oncology for several indications, including diagnosis, staging, response evaluation, targeting of radiation therapy, disease control and recurrence detection [1, 2]. Particularly, in malignant melanoma, [18F]FDG PET/CT is considered the modality of choice when there is clinical suspicion of locoregional or systemic metastases at initial staging, disease recurrence, and for treatment response assessment [3–7].
In most types of tumors, the imaging field of view (FOV) for PET/CT studies is from the skull base to upper thigh (torso imaging), which covers most of the relevant portions of the body in many oncological diseases [1]. However, due to the propensity of malignant melanoma to metastasize anywhere in the body, true whole-body (total body) imaging, i.e., from the top of the head through the feet, is recommended as the standard of care for the assessment of the malignancy [8]. This approach, however, leads to a non-negligible extension of the scanning time with a subsequent decrease in throughput, which is important for busy nuclear medicine departments.
Significant developments have occurred in the field of molecular imaging since the original description of the prototype, fused PET/CT scanner by Beyer et al. [9]. Most recently, long axial field of view (LAFOV) PET/CT systems, such as the Biograph Vision Quadra (Siemens Healthineers) and the uEXPLORER (United Imaging), have become available, rendering digital total-body PET/CT as the current state of the art, enabling, apart from the achievement of larger anatomical coverage, an increase in system sensitivity [10–12]. Indeed, preliminary results from the clinical application of these systems show that they lead to improved image quality and lesion quantification resulting from higher sensitivity, alternatively allowing for a significant reduction in acquisition time or low-dose examination protocols [13–15].
In the present study, we investigated melanoma patients with the new LAFOV Biograph Vision Quadra PET/CT after application of low-dose [18F]FDG. Our aim was to determine an appropriate acquisition time range for low-dose oncological imaging by analyzing different emulated scan times and the quality of the resultant PET images.
Materials and methods
Patients
A total of 49 patients (31 male, 18 female; mean age 64.9 years) with malignant melanoma were enrolled in this retrospective analysis. All patients underwent [18F]FDG PET/CT for staging or re-staging purposes. Seven patients underwent two PET/CT scans, while two of them underwent in total three scans. Thus, in total, 60 PET/CT scans were performed and analyzed. Patients gave written informed consent to participate in the study and to have their medical records released. The study was approved by the Ethical Committee of the University of Heidelberg (S-107/2012) and the Federal Agency for Radiation Protection (Bundesamt für Strahlenschutz, Z 5-22,463/2-2012-016). The patients’ characteristics are summarized in Table 1.
Table 1.
Baseline patient characteristics
| Patient characteristics | N |
|---|---|
| Number of patients | 49 |
| Total number of PET/CT scans | 60 |
| Median applied dose, MBq | 166 (93–263) |
| Median age, years | 64 (35–87) |
|
Gender Male Female |
|
| 31 (63%) | |
| 18 (37%) | |
| Type of primary melanoma | |
| cutaneous | 40 (82%) |
| mucosal | 1 (2%) |
| uveal | 5 (10%) |
| not defined | 3 (6%) |
| Previous systemic therapy | |
| yes | 26 (53%) |
| no | 23 (47%) |
| Tumor stage at the time of PET/CT | |
| I | 4 (8%) |
| II | 4 (8%) |
| III | 12 (24%) |
| IV | 26 (53%) |
| not defined | 3 (6%) |
PET/CT examination
All patients fasted for at least 6 h before [18F]FDG administration. Patients underwent total body PET/CT (Biograph Vision Quadra, Siemens Co., Erlangen, Germany) after intravenous administration of body weight-adapted dose of 2.0 MBq/kg [18F]FDG 60 min post-injection (p.i.). Total-body imaging from the skull through the feet was performed in two bed positions (each FOV 106 cm): the first bed position covered the area from the top of the head to the upper thigh (10-min acquisition in list mode; PET-10), and the second covered the lower extremities (5-min acquisition in list mode). The PET images of the first bed position (head to upper thigh) were first reconstructed using the entire 10-min data and were further split into 8-min (PET-8), 6-min (PET-6), 5-min (PET-5), 4-min (PET-4), and 2-min (PET-2) duration groups to compare different acquisition times for fast acquisition scenarios.
A low-dose attenuation CT (120 kV, 30 mA) was used for attenuation correction of the PET data and for image fusion. All PET images were attenuation-corrected and an image matrix of 440 × 440 pixels was used for iterative image reconstruction. Images were reconstructed using the manufacturer’s (Siemens Healthineers) default reconstruction method, employing the point spread function + time-of-flight algorithm (PSF + TOF, 4 iterations × 5 subsets) without Gaussian filtering into 1.65 × 1.65 × 1.65 mm3 voxels.
Data analysis
Visual assessment of PET/CT scans
PET/CT images were evaluated independently on an Aycan workstation by two experienced nuclear medicine physicians well versed in melanoma diagnosis (CS, ADS). In cases of discrepancy, an agreement was reached after mututal consultation. In order to reduce bias, with regard to the same examination, the reading of PET/CT scans for the different time acquisition protocols was performed with an interval of at least 1 week.
Visual analysis was based on the identification of sites of focal, non-physiologic [18F]FDG uptake above surrounding background activity, which were considered consistent with melanoma lesions. On the basis of these findings, each scan was classified as normal (no melanoma lesions) or pathologic (at least one [18F]FDG-avid melanoma lesion). Moreover, the number of melanoma lesions was determined in each scan, with a maximum of up to 10 measured lesions per patient. With regard to lesion detectability, the results of the 10-min PET acquisition (PET-10) served as the reference, with which the results of the other duration groups (PET-8, PET-6, PET-5, PET-4, PET-2) were compared.
Objective evaluation of PET/CT image quality
Objective evaluation of PET/CT image quality was based on volumes of interest (VOIs) and on subsequent calculation of SUV values (SUVmean, SUVmax). Melanoma lesions’ quantification was based on VOIs drawn with an isocontour mode (pseudo-snake) that were placed over tumor lesions using a dedicated software (PMOD Technologies, Zurich, Switzerland) [16]. With regard to melanoma lesions, these were assessed on a patient-basis (‘patient-based analysis’), calculated from all evaluated [18F]FDG-avid lesions in each PET/CT scan and averaged in each patient, and on an independent-basis (lesion-based analysis), in which each tumor lesion was evaluated separately.
Due to its reasonably uniform tracer uptake, the liver parenchyma was used for background measurements by the positioning of spheric VOIs in the right liver lobe, if without lesions, and at least 1 cm away from the edge of the liver [1, 17]. Moreover, semi-quantitative evaluations of the blood pool were performed after placement of VOIs in the lumen of the descending aorta in the mediastinum without inclusion of the aortic wall. VOIs were copied and pasted between different images obtained from different (list mode) frame durations, ensuring that the same VOI was analysed for each acquisition as previously described [14]. The signal-to-noise ratio (SNR) of the background (liver) was measured as the SUVmean of the background divided by its standard deviation (SD). Tumour-to-background ratios (TBRs) were defined as SUVmean of the melanoma lesion divided by SUVmean for the liver background [14, 17].
It has to be noted that the comparison of the results, both of visual analysis and objective evaluation of PET/CT image quality, was focused on the first bed position covering the area from the top of the head to the upper thigh.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD). Regarding visual (qualitative) assessment of PET/CT scans, score-based 95%-confidence intervals (95%-CI) for the differences of the proportion of pathological scans between the various PET acquisition times and the reference of PET-10 were calculated using the method of Tango and Tang as implemented in the function pairbinci in the R package ratesci [18–20]. Respectively, concerning the lesion detection rate, the 95%-CI for the 90%-quantile of the differences in number of tumor lesions between the various PET acquisition times and the reference of PET-10 were calculated using the function quantileCI from the R package MKinfer. Further, differences between parameters employed for objective evaluation of PET/CT image quality in the liver and mediastinum were assessed using the paired Student’s t-test. For the tumor lesions, in terms of lesion-based analysis the differences were assessed using a linear mixed model to take into account that some lesions belonged to the same patient, with log transformation employed on the quantitative parameters to eliminate skewness observed in the measurements. Respectively, for patient-based analysis, differences were assessed using paired t-test after averaging the lesions measurements for each patient. The different PET/CT scans of some patients examined in different time points (n = 11 scans of seven patients) were considered independent in this analysis, since in the meantime the patients had received systemic therapies, which markedly altered the [18F]FDG uptake. Statistical significance was considered for p-values less than 0.05. Statistical analysis was performed in R (version 4.0.3).
Results
Visual assessment of PET/CT scans
Based on the results of PET-10, a total of 49/60 pathological (81.7%) and 11 normal (18.3%) scans were diagnosed. The respective numbers of pathological PET/CT scans were 49/60 (81.7%) for PET-8, 49/60 (81.7%) for PET-6, 48/60 (80%) for PET-5, 46/60 (76.7%) for PET-4, and 45/60 (75%) for PET-2. The 95%-CI for the differences of proportion of the pathological scans among the different, shorter PET acquisitions and the reference of PET-10 were the following: − 0.0602–0.0602 (PET-8), − 0.0602–0.0602 (PET-6), − 0.0445–0.0886 (PET-5), − 0.0132–0.1370 (PET-4), and 0.0025–0.1593 (PET-2).
In 18 PET-10 scans, the extent of hypermetabolic metastatic involvement was very large (> 10 lesions), rendering the exact calculation of [18F]FDG-avid tumor lesions very difficult. In these 18 cases, a very large number of lesions (> 10) was also detected with all rest reconstruction protocols, with the exception of one case, in which PET-2 demonstrated less than ten, in paticular eight, tumor lesions. In the remaining 42 PET-10 scans, for which the exact calculation of tumor lesions was feasible, a total of 119 lesions was detected. Compared to the reference of PET-10 images, PET-8, PET-6, PET-5, PET-4, and PET-2 images had lesion detection rates of 97.5% (116/119), 95.0% (113/119), 89.9% (107/119), 83.2% (99/119), and 73.9% (88/119), respectively. The 95%-CI for the 90%-quantile of the differences in number of tumor lesions between the various PET acquisition times and the reference of PET-10 were as follows: 0–1 (PET-8), 0–1 (PET-6), 0–2 (PET-5), 1–2 (PET-4), and 2–4 (PET-2). Notably, the observed differences between PET-10, PET-8, and PET-6 regarding the number of detected lesions, would have no clinical or therapeutic consequences for any of the studied patients, since they would not potentially lead to differences in terms of staging or restaging the disease.
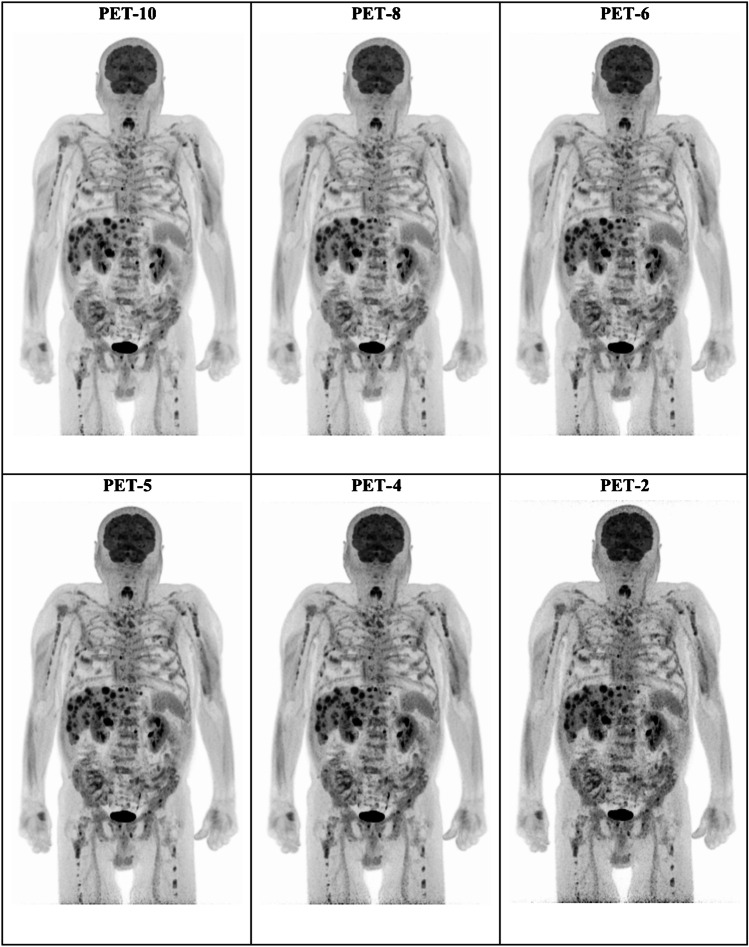
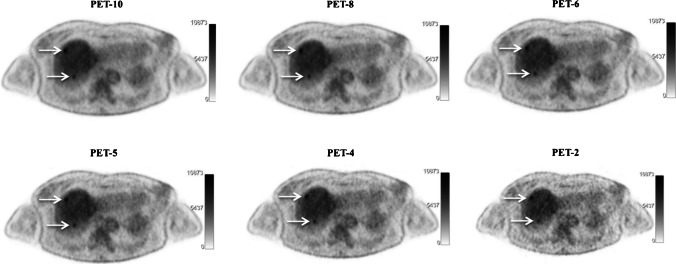
The results of the visual assessment of PET/CT scans are presented in Tables 2 and 3. Figures 1 and 2 present examples of [18F]FDG PET images of patients assessed with different time acquisition protocols.
Table 2.
Results of visual analysis of PET/CT images with regard to classification of scans as normal (no melanoma lesions) or pathologic (at least one [18F]FDG-avid melanoma lesion)
| Measurement | PET-10 | PET-8 | PET-6 | PET-5 | PET-4 | PET-2 |
|---|---|---|---|---|---|---|
| Number of pathological scans | 49/60 (81.7%) | 49/60 (81.7%) | 49/60 (81.7%) | 48/60 (80.0%) | 46/60 (76.7%) | 45/60 (75.0%) |
| 95%-CI for difference of proportions of pathological scans# | n.a | -0.0602—0.0602 | -0.0602—0.0602 | -0.0445—0.0886 | -0.0132—0.1370 | 0.0025—0.1593 |
#Compared to the reference of PET-10
n.a. not applicable
Table 3.
Results of visual analysis of PET/CT images with regard to lesion detection rate
| Measurement | PET-10 | PET-8 | PET-6 | PET-5 | PET-4 | PET-2 |
|---|---|---|---|---|---|---|
| Lesion detection rate # | n.a | 116/119 (97.5%) | 113/119 (95.0)% | 107/119 (89.9%) | 99/119 (83.2%) | 88/119 (73.9%) |
| 95%-CI for 90%-quantile of differences of number of lesions # | n.a | 0–1 | 0–1 | 0–2 | 1–2 | 2–4 |
After exclusion of the 18 scans in which the exact calculation of tumor lesions was very difficult due to extent of metastatic involvement (> 10 lesions)
n.a. not applicable
#Compared to the reference of PET-10
Fig. 1.
Example of maximum intensity projection (MIP) [18F]FDG PET images of a 74-year-old man with stage IV cutaneous melanoma planned for treatment with immune checkpoint inhibitors. Presented are the PET-10, PET-8, PET-6, PET-5, PET-4, and PET-2 acquisitions. All acquisitions clearly demonstrate disseminated [18F]FDG-avid metastastic disease
Fig. 2.
Example of axial [18F]FDG PET images of a 61-year-old woman with stage IV uveal melanoma under treatment with tebentafusp. Presented are the PET-10, PET-8, PET-6, PET-5, PET-4, and PET-2 acquisitions. Two focal hypermetabolic, metastatic lesions in the upper part of the liver parenchyma (arrows) clearly depicted in the PET-10, PET-8, PET-6, PET-5, and PET-4 acquisitions. The lesions are, on the other hand, not very distinct in the PET-2 acquisitions, especially the dorsally localized metastasis
Objective evaluation of PET/CT image quality
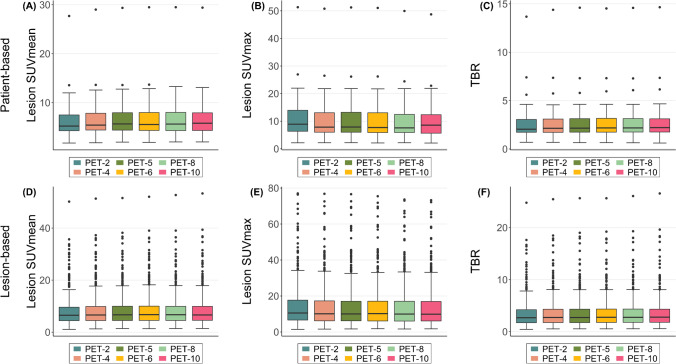
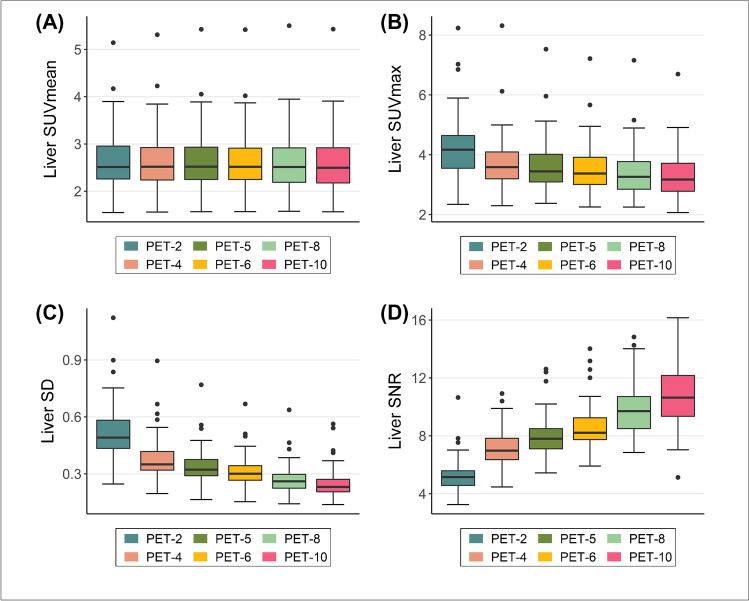
The results of the objective image quality assessments are presented in Figs. 3, 4, and 5 as well as in Supplementary File 1. As a general trend, as the acquisition time decreased the background (liver) uptake and image noise increased, while the liver SNR decreased. In particular, the liver SNR calculated in the PET-10 images was significantly higher than all other acquisitions. Similarly, tracer uptake in the mediastinum progressively increased as PET acquisition duration decreased.
Fig. 3.
Box plots for comparison of objective image quality parameters in tumor lesions among the different PET acquisition groups in terms of patient-based (A–C) and lesion-based (D–F) analysis. As a general trend, SUVmean decreased while moving from longer to shorter-duration protocols, although the changes with the reference 10-min values were non-significant up to 5-min acquisitions (A, C). SUVmax was significantly lower in PET-10 than all other shorter-duration protocols (B, D). TBR decreased in shorter protocols, although the decrease was non-significant up to 8-min and 6-min acquisitions in patient- and lesion-based analysis respectively (C, F)
Fig. 4.
Box plots for comparison of objective image quality parameters in the liver. As a general trend, the liver uptake, SD and image noise progressively increased (A–C) while the liver SNR decreased (D) as acquisition times decreased
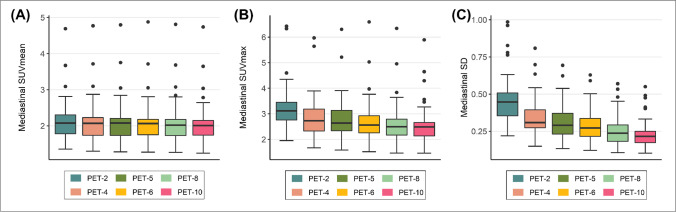
Fig. 5.
Box plots for comparison of objective image quality parameters in the mediastinum (descending aorta). As a general trend, the blood pool uptake and SD progressively increased as acquisition times decreased (A–C)
A total of 463 tumor lesions were evaluated quantitavely. Both patient-based and lesion-based analysis revealed for SUVmean no significant differences between 10-min, 8-min, 6-min, and 5-min acquisitions, while in shorter-duration protocols (PET-2, PET-4) SUVmean decreased significantly. Respectively, SUVmax was significantly lower in PET-10 than all shorter-duration protocols in both patient-based and lesion-based analysis. Finally, the shortening of acquisition time up to 6 min and 8 min led to no differences regarding TBR in lesion- and patient-based analysis respectively, while in shorter-duration protocols TBR significantly decreased compared to the 10-min protocol.
Discussion
In standard clinical practice, the achievement of high quality PET images that can overcome the inherent poor sensitivity of current whole-body PET scans requires either an increase in the radiotracer injection dose or a prolongation of the acquisition time, both methods with radiation exposure- and logistical consequences [15, 21]. In this context, the recent advent and clinical introduction of ultra-extended FOV PET/CT scanners represents an evolution in molecular imaging with potentially major clinical and financial implications, while enabling, at the same time, a reduction of the associated radiation burden [22]. Importantly, it has already been shown that low-dose or even ultra-low-dose radiotracer activities achieve a comparable image quality to conventional PET, and meet clinical requirements in different tumors [13–15, 23, 24]. Based on this experience, we, herein, aimed to determine an appropriate acquisition time range for low-dose oncological PET/CT imaging with the new LAFOV Biograph Vision Quadra PET/CT (Siemens Healthcare) after application of 2.0 MBq/kg, and not 3.0 MBq/kg as per EANM guidelines [1], [18F]FDG activity in a cohort of melanoma patients.
The major findings of our analysis are the following: firstly, the reduction of PET acquisition times up to 5 min in low-dose [18F]FDG LAFOV PET/CT imaging is feasible in melanoma and can be safely performed in terms of visual analysis, applied both in a binary fashion (normal-pathological) and based on calculation of lesion detection rate. In particular, the shortening of PET acquisition times to 6 min was associated with absolutely no potential clinical consequences in the context of patient staging or restaging in the present cohort for any of the studied patients. Further, a decrease of both SNR and TBR is observed by reducing the PET acquisition time, although the change in absolute tumor SUVmean values is small and clinically rather non-relevant.
The Biograph Vision Quadra PET/CT system essentially is comprised of an axial concatenation of the equivalent of four Biograph Vision PET 600 scanners, providing an axial FOV of 106 cm, equipped with silicon-based photomultiplier (SiPM)-based photon detectors characterised by superior timing resolution. These characteristics enable improved TOF estimation and efficient photon detection, a high spatial resolution, and an increased sensitivity by a factor of five when compared to the preceding Biograph Vision 600 system [25–28]. The results of the visual analysis of our study show that this increased sensitivity of the new scanner, may allow for a reasonable decrease in acquisition time by 5 min (i.e., from 10- to 5-min), even when applying low dose examination protocols. Further, absolutely no differences were observed between the reference of 10-min PET protocol and the 8-min and 6-min acquisitions regarding classification of scans as normal or pathological, while the differences in lesion detectability rate were minimal and, importantly, without any potential clinical consequence for any patient of the cohort. This means that all patients would have been correctly characterized in terms of staging and restaging of the disease even when applying a 6-min, low-dose PET/CT protocol. Notably, with regard to the proportion of pathological scans, a decrease of the acquisition times even up to four minutes did not lead to any significant change, as reflected by the respective 95%-CI for the differences between various acquisition prortocols.
Particularly in malignant melanoma, where true whole-body (total body) imaging and, therefore, two-bed position scanning with the Biograph Vision Quadra system is required, this shortening of PET acquisition times without a concomitant, clinically relevant decrease of diagnostic performance would be very practical for busy departments, since it would allow the execution of a greater number of exams and lead to an increase in patient throughput. At the same time, the shortened protocol would improve patient comfort and considerably prevent motion artifacts. Moreover, the reduction of the applied radiopharmaceutical dose represents an economic as well as a dosimetric advantage. These considerations take on even greater significance, when taking into account the potential need for serial PET/CT scanning of many of the patients with advanced melanoma who undergo immunotherapy in terms of treatment monitoring [29].
On the other side, the shortening of the PET protocol was associated with a decrease of both parameters used to objectively evaluate image quality, namely SNR and TBR. The observation that extending the acquisition times improves image quality is not new. In specific, the herein observed trend is in line with the results of previous studies also employing ultra-extended FOV PET/CT scanners, which similarly showed a decreasing SNR as PET acquisition times decreased in cohorts of different tumors studied with different tracers applied both at standardized and low doses [13–15].
The parameter TBR also decreased progressively from PET-10 to PET-2. This finding seems to be inconsistent with previous studies on ultra-extended FOV PET/CT scanners, which showed an inverse relationship, i.e., higher TBR values in shorter acquisitions compared to standard acquisitions [13, 15]. It has to be noted, however, that in these studies, TBR was calculated as the ratio of SUVmax of tumor lesions divided by SUVmean of the liver background, which was not the case in our analysis. In the present analysis TBR was defined as the ratio of SUVmean of the melanoma lesions divided by SUVmean of the liver. We prefered to calculate the mean tumor uptake since it incorporates information from multiple voxels, making it less susceptible to image noise compared to SUVmax [30]. This approach of SUVmean-based calculation of TBR was also recently applied by Sari et al. in another study with the Biograph Vision Quadra system [31]. Indicatively, the application of the alternative formula (lesion SUVmax/liver SUVmean) would have led to consistent results with the above-mentioned studies, namely a progressive increase of TBR as the acquisition times decrease (data not provided in the manuscript but available at request). Notably, in lesion-based analysis, the reduction of acquisition time to 6 min and 5 min led to no significant differences in TBR and tumor lesions’ SUVmean, respectively.
Our study has some limitations. Firstly, this is a single-center retrospective analysis. Thus, a validation of the herein presented findings in larger patient cohorts ideally studied in the context of a multicenter, prospective trial would be required. Secondly, the use of histopathological findings as a reference to validate the detectability of tumor lesions is a more reliable method than referring to PET-10 images. However, in our study the vast majority of the PET-positive findings were not histopathologically confirmed, which is, obviously, not possible in the clinical setting and was, moreover, beyond the scope of this analysis. Nevertheless, since all included patients had a biopsy-confirmed melanoma, for which they received the respective treatment, and given the very high accuracy of [18F]FDG PET/CT in detection of the malignancy, most focal [18F]FDG-avid lesions, for which a benign aetiology could be safely excluded, were considered consistent with tumor involvement. Thirdly, the analysis was confined to the comparison of the 10-min PET scans, as recommended by the manufacturer, with five specific sub-divisions (2-min, 4-min, 5-min, 6-min, and 8-min) of the original acqusitions, and did not include even more acquisitions. However, we believe that the conclusions drawn with the present comparisons provide sufficient hints for clinical practice in the context of the purpose of this study. Finally, the study focused on the analysis of the scans covering the first bed position, i.e. from the head to the thigh. The PET scans performed in terms of the second bed position, covering the lower extremities, were not analyzed in the same fashion, since the original PET acquisitions lasted shorter (5 min). This will be however the topic of a future work of our group.
Conclusion
In an attempt to determine an appropriate acquisition time range for low-dose oncological imaging with the new LAFOV Biograph Vision Quadra PET/CT, we investigated a cohort of malignant melanoma patients after administration of body weight-adapted dose of 2.0 MBq/kg [18F]FDG. We could show that the reduction of PET acquisition times from head to upper thigh up to 5 min in low-dose LAFOV PET/CT imaging can be safely performed, leading to no significant changes in terms of visual analysis. Moreover, the shortening of the PET protocol from 10 to 6 min was associated with absolutely no potential clinical consequences for any of the enrolled patients. Finally, an increase in noise was observed by reducing the protocol from longer to shorter acquisition times, although in lesion-based analysis, the change in TBR and tumor SUVmean values was non-significant up to 6-min and 5-min acquisitions, respectively. These findings may have significant implications for the diagnostic work-up of patients with melanoma, given the need for true whole-body imaging in this type of cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all participants enrolled in the study.
Consent for publication
Consent to publish has been received from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Oncology - General.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT EANM procedure guidelines for tumour imaging version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. [DOI] [PMC free article] [PubMed]
- 2.Petersen H, Holdgaard PC, Madsen PH, Knudsen LM, Gad D, Gravergaard AE, Rohde M, Godballe C, Engelmann BE, Bech K, Teilmann-Jørgensen D, Mogensen O, Karstoft J, Johansen J, Christensen JB, Johansen A, Høilund-Carlsen PF; PET/CT Task Force of the Region of Southern Denmark. FDG PET/CT in cancer: comparison of actual use with literature-based recommendations. Eur J Nucl Med Mol Imaging. 2016 Apr;43(4):695–706. 10.1007/s00259-015-3217-0. [DOI] [PMC free article] [PubMed]
- 3.Rodriguez Rivera AM, Alabbas H, Ramjaun A, Meguerditchian AN. Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta-analysis. Surg Oncol 2014;23:11–16. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24556310. Accessed 21 Sept 2022. [DOI] [PubMed]
- 4.Schule SC, Eigentler TK, Garbe C, et al. Influence of (18)F-FDG PET/CT on therapy management in patients with stage III/IV malignant melanoma. Eur J Nucl Med Mol Imaging 2016;43:482–488. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26384681. Accessed 21 Sept 2022. [DOI] [PubMed]
- 5.Hofman MS, Hicks RJ Role of PET/CT in Melanoma. In: PET/CT in Melanoma. Hofman MS, Hicks RJ (eds). Springer International Publishing AG 2017.
- 6.Sachpekidis C, Dimitrakopoulou-Strauss A. Melanoma: 18F-FDGPET/CT for response assessment of melanoma following immunotherapy. In: Lopci E, Fanti S, editors. Atlas of Response to Immunotherapy. Cham: Springer; 2020.
- 7.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Melanoma: Cutaneous Version 3.2022 — April 11, 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed: September 22, 2022.
- 8.Coleman RE, Delbeke D, Guiberteau MJ, Conti PS, Royal HD, Weinreb JC, Siegel BA, Federle MF, Townsend DW, Berland LL; American College of Radiology; Society of Nuclear Medicine; Society of Computed Body Tomography and Magnetic Resonance. Concurrent PET/CT with an integrated imaging system: intersociety dialogue from the joint working group of the American College of Radiology, the Society of Nuclear Medicine, and the Society of Computed Body Tomography and Magnetic Resonance. J Nucl Med. 2005 Jul;46(7):1225–39. [PubMed]
- 9.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 10.Badawi RD, Shi H, Hu P, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60:299–303. doi: 10.2967/jnumed.119.226498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberghe S, Moskal P, Karp JS. State of the art in total body PET. EJNMMI Phys. 2020;7(1):35. doi: 10.1186/s40658-020-00290-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel S, Aykac M, Bal H, et al. Preliminary performance of a prototype, one-meter long PET tomograph. Paper presented at: 2020 IEEE Nuclear Science Symposium and Medical Imaging Conference, Virtual; Boston, Massachusetts; November 5, 2020.
- 13.Tan H, Sui X, Yin H, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(6):1966–1975. doi: 10.1007/s00259-020-05091-4. [DOI] [PubMed] [Google Scholar]
- 14.Alberts I, Hünermund JN, Prenosil G, Mingels C, Bohn KP, Viscione M, Sari H, Vollnberg B, Shi K, Afshar-Oromieh A, Rominger A. Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur J Nucl Med Mol Imaging. 2021 Jul;48(8):2395–2404. doi: 10.1007/s00259-021-05282-7. [DOI] [PMC free article] [PubMed]
- 15.He Y, Gu Y, Yu H, Wu B, Wang S, Tan H, Cao Y, Chen S, Sui X, Zhang Y, Shi H. Optimizing acquisition times for total-body positron emission tomography/computed tomography with half-dose 18F-fluorodeoxyglucose in oncology patients. EJNMMI Phys. 2022;9(1):45. doi: 10.1186/s40658-022-00474-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.pmod.com/files/download/v31/doc/pbas/4729.htm
- 17.Jakoby BW, Bercier Y, Conti M, Casey ME, Bendriem B, Townsend DW. Physical and clinical performance of the mCT time-of-flight PET/CT scanner. Phys Med Biol. 2011;56:2375–2389. doi: 10.1088/0031-9155/56/8/004. [DOI] [PubMed] [Google Scholar]
- 18.Tango T. Equivalence test and confidence interval for the difference in proportions for the pairedsample design. Stat Med. 1998;17:891–908. doi: 10.1002/(SICI)1097-0258(19980430)17:8<891::AID-SIM780>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Tango T. Improved confidence intervals for the difference between binomial proportions based on paired data by Robert G. Newcombe, Statistics in Medicine, 17, 2635–2650 (1998). Statistics in Medicine 1999; 18(24):3511–3513. [DOI] [PubMed]
- 20.Tang N-S, Tang M-L, Chan ISF. On tests of equivalence via non-unity relative risk for matched-pair design. Stat Med. 2003;22:1217–1233. doi: 10.1002/sim.1213. [DOI] [PubMed] [Google Scholar]
- 21.Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J Nucl Med. 2018 Jan;59(1):3–12. 10.2967/jnumed.116.184028. [DOI] [PMC free article] [PubMed]
- 22.Hicks RJ, Van den Abbeele AD. Will ultra-extended field-of-view scanners be an expensive folly or the next clinical standard for PET/CT? Cancer Imaging. 2022;22(1):49. doi: 10.1186/s40644-022-00486-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Liu G, Yu H, Wang Y, Li C, Tan H, Chen S, Gu J, Shi H. Feasibility of Acquisitions Using Total-Body PET/CT with an Ultra-Low 18F-FDG Activity. J Nucl Med. 2022 Jun;63(6):959–965. doi: 10.2967/jnumed.121.262038. [DOI] [PMC free article] [PubMed]
- 24.Alberts I, Schepers R, Zeimpekis K, Sari H, Rominger A, Afshar-Oromieh A. Combined [68 Ga]Ga-PSMA-11 and low-dose 2-[18F]FDG PET/CT using a long-axial field of view scanner for patients referred for [177Lu]-PSMA-radioligand therapy. Eur J Nucl Med Mol Imaging. 2022 Sep 22. doi: 10.1007/s00259-022-05961-z. [DOI] [PMC free article] [PubMed]
- 25.van Sluis J, de Jong J, Schaar J, Noordzij W, van Snick P, Dierckx R, Borra R, Willemsen A, Boellaard R. Performance Characteristics of the Digital Biograph Vision PET/CT System. J Nucl Med. 2019;60(7):1031–1036. doi: 10.2967/jnumed.118.215418. [DOI] [PubMed] [Google Scholar]
- 26.Prenosil GA, Sari H, Fürstner M, Afshar-Oromieh A, Shi K, Rominger A, Hentschel M. Performance characteristics of the biograph vision quadra PET/CT system with a long axial field of view using the NEMA NU 2–2018 standard. J Nucl Med. 2022;63(3):476–484. doi: 10.2967/jnumed.121.261972. [DOI] [PubMed] [Google Scholar]
- 27.Prenosil GA, Hentschel M, Weitzel T, Sari H, Shi K, Afshar-Oromieh A, Rominger A. EARL compliance measurements on the biograph vision Quadra PET/CT system with a long axial field of view. EJNMMI Phys. 2022;9(1):26. doi: 10.1186/s40658-022-00455-1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Sluis J, van Snick JH, Brouwers AH, Noordzij W, Dierckx RAJO, Borra RJH, Slart RHJA, Lammertsma AA, Glaudemans AWJM, Boellaard R, Tsoumpas C. EARL compliance and imaging optimisation on the Biograph Vision Quadra PET/CT using phantom and clinical data. Eur J Nucl Med Mol Imaging. 2022 Jul 25. 10.1007/s00259-022-05919-1. [DOI] [PMC free article] [PubMed]
- 29.Lopci E, Hicks RJ, Dimitrakopoulou-Strauss A, Dercle L, Iravani A, Seban RD, Sachpekidis C, Humbert O, Gheysens O, Glaudemans AWJM, Weber W, Wahl RL, Scott AM, Pandit-Taskar N, Aide N. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging. 2022 Jun;49(7):2323–2341. 10.1007/s00259-022-05780-2. [DOI] [PMC free article] [PubMed]
- 30.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010 Aug;195(2):310-20. 10.2214/AJR.10.4923. Erratum in: AJR Am J Roentgenol. 2010 Oct;195(4):1043. [DOI] [PubMed]
- 31.Sari H, Mingels C, Alberts I, Hu J, Buesser D, Shah V, Schepers R, Caluori P, Panin V, Conti M, Afshar-Oromieh A, Shi K, Eriksson L, Rominger A, Cumming P. First results on kinetic modelling and parametric imaging of dynamic 18F-FDG datasets from a long axial FOV PET scanner in oncological patients. Eur J Nucl Med Mol Imaging. 2022;49(6):1997–2009. doi: 10.1007/s00259-021-05623-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.