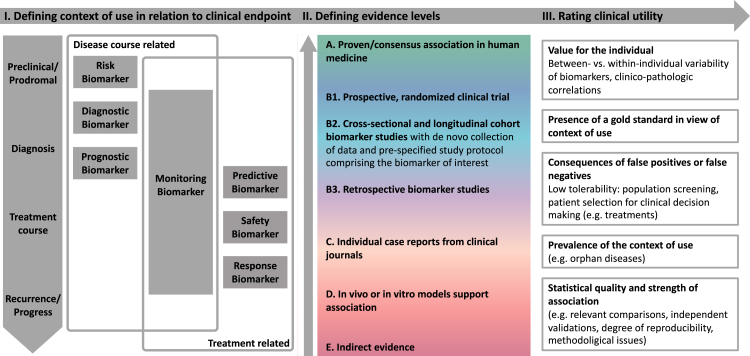
Fig. 1.
Unifying classification concept for protein biomarkers across neurologic disease entities. According to the unified classification, a biomarker is first classified by its clinical application by grouping it into one of the seven categories and by precisely defining the associated clinical endpoint. In a second step, the available evidence is summarized in one of the levels A–E. Rating clinical utility in a third step is difficult to operationalise. Depending on the individual patient case and disease, the one or other approach may be more suitable. It is important to note whether a gold-standard for the measurement of the clinical endpoint already exists or whether there is a general lack of appropriate biomarkers.