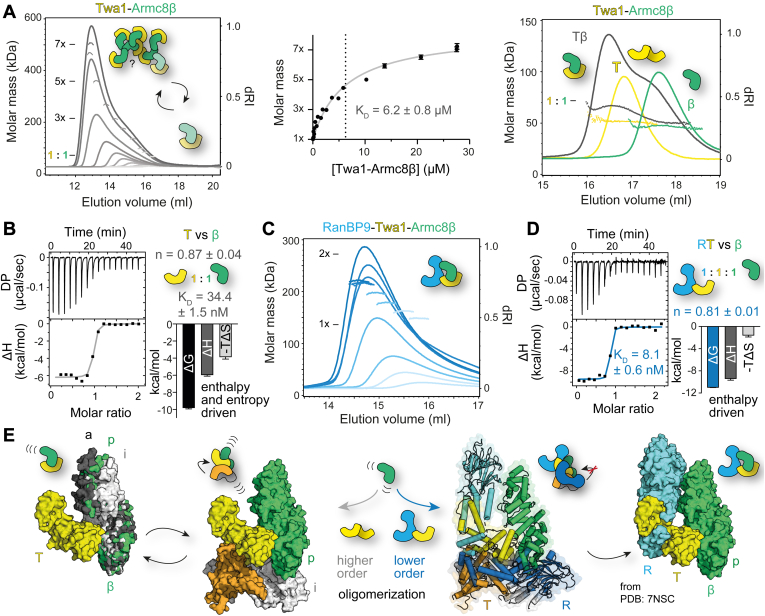
Figure 5.
Tight binding to RanBP9 prevents higher order oligomerization of the Twa1–Armc8β complex.A, SEC–MALS analysis of the Tβ complex self-association conducted as in Figures 2B and 4A. Full molar mass peak profiles are shown in Fig. S1B. In addition, assembly and molecular weight analysis of the binary complex at low concentrations are depicted (right). B, ITC binding studies of Twa1 to Armc8β displayed as for Figure 3C. The affinity constant KD and signature binding plot parameters including their standard errors were derived from 18 measurements. C, molecular weight determination of RTβ complex self-association with SEC–MALS at different concentrations was limited by the instability of the complex at higher concentrations. See Fig. S1C for full molar mass profiles. D, ITC analysis of the RT complex binding to Aβ derived from seven measurements. E, model of how the oligomerization of the binary Tβ complex is prevented upon addition of RanBP9. To demonstrate this the Twa1-Armc8α module from the RTα–Gid4 complex (PDB entry: 7NSC) was superimposed with the three Armc8β conformations observed in the crystal structure. ITC, isothermal titration calorimetry; SEC-MALS, multiangle light scattering coupled to analytical size exclusion chromatography; PDB, Protein Data Bank.