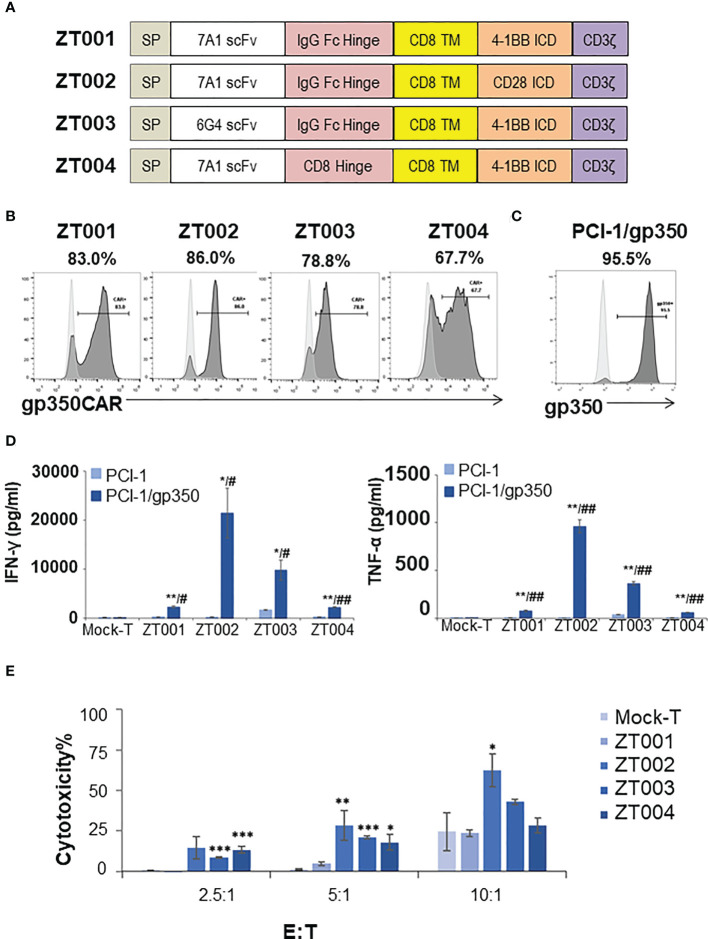
Figure 1.
Testing of different lentiviral vectors incorporating different gp350CAR designs and selection of the best construct. (A) Schematic diagrams of LV constructs expressing gp350CAR. SP, signal peptide; scFv, single chain variable fragment (7A1 or 6G4); Hinge (IgG Fc or CD8); TM, transmembrane domain (all CD8); ICD, intracellular domain (CD28.CD3ζ or 4-1BB.CD3ζ). (B) Flow cytometry analyses showing surface expression of the CAR on T cells transduced with four lentiviral constructs (ZT001, ZT002, ZT003 or ZT004). Transduced T cells (dark grey) are compared with mock-T cells (light grey). Percentages of gp350CAR-expressing cells are shown; representative example from triplicate transduction experiments performed with T cells derived from one donor. (C) Flow cytometry analyses of PCI-1 cells transduced to express gp350 (dark grey) compared with un-transduced cells (light grey). (D) IFN-γ and TNF-α release. Mock-T and gp350 CAR-T cells were co-cultured with PCI-1 or PCI-1/gp350 cells for 16 hours at an E:T of 5:1 and the levels of IFN-γ and TNF-α released in the medium were measured by ELISA. Data are presented as means from triplicates ± SD. Welch t-test and p-values after correction for multiple comparisons, CAR-T versus Mock-T cells, *P ≤0.05, **P≤0.01, ***P ≤0.001. PCI-1/gp350 versus PCI-1, #P ≤0.05, ##P≤0.01. (E) In vitro cytotoxicity comparison of four anti-gp350 CAR. Lactose dehydrogenase (LDH)-based cytotoxicity assay (16 hours culturing) was used to assess the cytotoxicity of four anti-gp350 CAR-T cells against gp350-positive human oropharyngeal cancer cell lines PCI-1 (PCI-1/gp350). Non-transduced T (Mock-T) cells were included as a control. These results are presented as means from triplicates ± s.d. Welch t-test and p-values after correction for multiple comparisons, compared to Mock-T, *, P < 0.05; **, P<0.01; ***, P < 0.001.