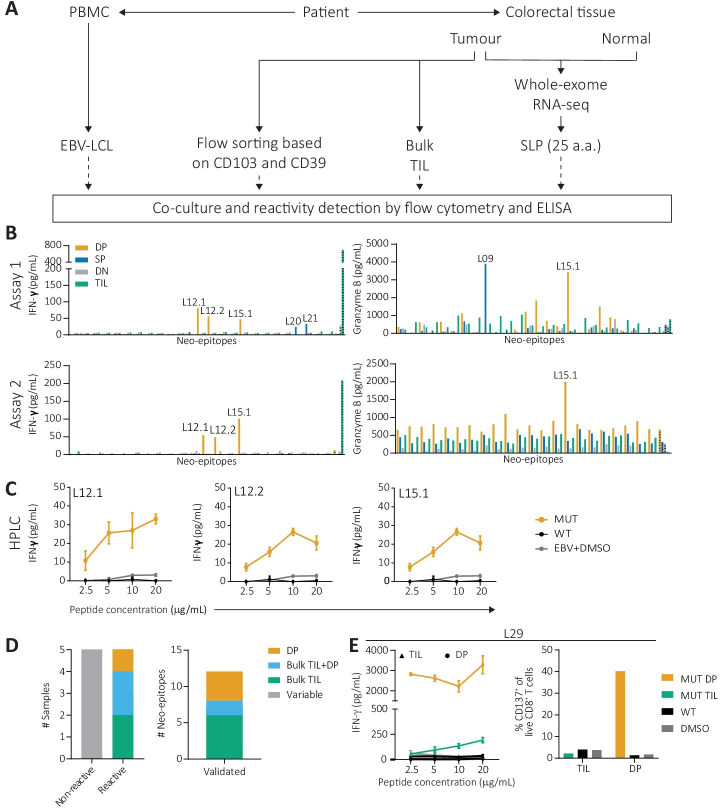
Figure 1.
Neoantigen-directed T cell reactivity assessment from bulk TIL and sorted CD8+ T cell subsets according to CD39 and CD103 expression. (A) Schematic workflow of the experimental setup. (B) A representative example of the IFN-γ and granzyme B ELISA measurements obtained in two independent assays performed in NIC16. Potential neoepitopes are depicted with the peptide number, for example, ‘L12.1’. (C) Representative example of a validation experiment in NIC16. The differential IFN-γ production upon coculture with the mutant peptide (yellow), the corresponding wild-type peptide (black) or a DMSO control (grey) was assessed in a peptide titration series ranging from 2.5 to 20 µg/mL. (D) Summary of the number of patient samples in which no reactivity was detected (gray), or with T cell responses derived from the DP subset (yellow), bulk TIL (green) or both the bulk TIL and DP subset (light blue). (E) IFN-γ production (left) and CD137 expression (right) on coculture of NIC4 bulk TIL (green) and DP subset (yellow) with the L29 epitope and controls. DP, double-positive; EBV-LCL, Epstein-Barr virus-transformed lymphoblastoid B cell lines; PBMC, peripheral blood mononuclear cell; TIL, tumor-infiltrating lymphocyte.