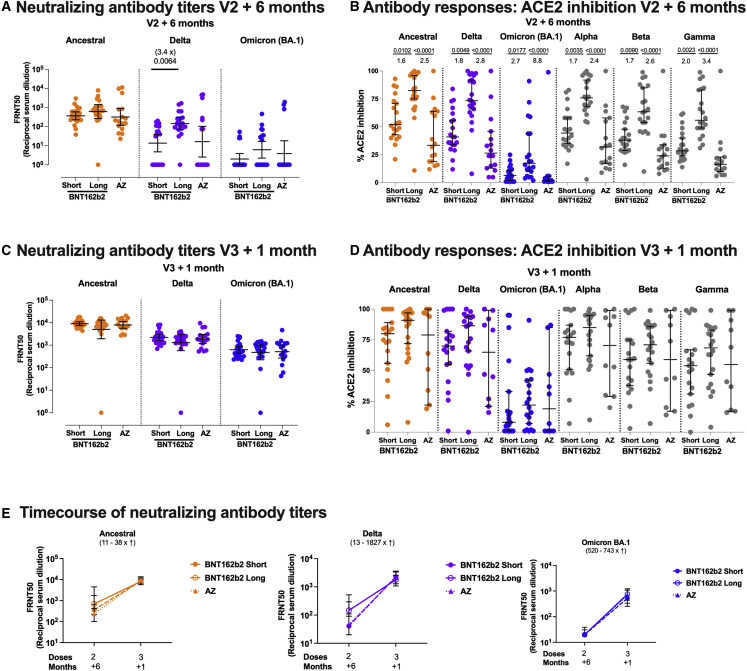
Figure 4.
Neutralizing antibody and ACE2 inhibition titer profiles against SARS-CoV-2 variants of concern 6 months after two doses of BNT162b2 or AZD1222 and 1 month after a third vaccine with BNT162b2
(A and C) Focus reduction neutralization assay 50% (FRNT50) antibody titers against the Victoria isolate (orange), Delta (B.1.617.2, purple), and Omicron BA.1 (B.1.1.529 BA.1, blue) taken from infection-naive participants. FRNT50 is the reciprocal dilution of the concentration of serum required to produce a 50% reduction in infectious focus-forming units of virus in Vero cells (ATCC CCL-81). Participants either received two doses of BNT162b2 (Pfizer-BioNTech) vaccine delivered in a short (3–5 weeks, n = 20) or long (6–17 weeks, n = 20) dosing interval or two doses of AZD1222 (AstraZeneca) vaccine (AZ, n = 16). Neutralizing antibody titers are shown in (A) 6 months after the second dose and, for the same individuals, (C) 1 month after a third booster dose of mRNA vaccine for all participants. Geometric mean neutralizing titers with 95% confidence intervals are shown.
(E) Comparison of the data from (A) and (C), plotted as means with error bars by vaccine regimen 6 months after the second vaccine (V2 + 6 months), 1 month after the third booster mRNA vaccine (V3 + 1 month). The range of fold change (median) between V2 + 6 months and V3 + 1 month for the three vaccine regimens (short, dashed line; long, solid line; and AZ, dotted line) is shown in brackets for each variant. Points represent the median, and error bars represent the IQR. Data in (A), (C), and (E) from the short group (n = 20) have been previously published.35
(B and D) Impact of short or long BNT162b2 vaccine dosing interval and AZ on the ability of sera to inhibit ACE2 binding to SARS-CoV-2 spike (Victoria isolate, Delta (B.1.617.2), Omicron BA.1 (B.1.1.529 BA.1), Alpha (B.1.1.7), Beta (B.1.351), and Gamma (P.1)) (B) 6 months after the second dose and (D) 1 month after a third booster dose with mRNA vaccine. ACE2 inhibition was analyzed using a multiplexed MSD assay and performed at a serum dilution of 1:10 at V2 + 6 months and 1:100 at V3 + 1 month. Data are shown as percentage of inhibition. Bars represent the median with 95% confidence intervals. Naive, short, n = 20; naive, long, n = 20; naive, AZ, n = 16 for V2 + 6 months; naive, short, n = 19; naive, long, n = 20; naive, AZ, n = 10 for V3 + 1 month. Vaccine regimens were compared with the Kruskal-Wallis nonparametric test and Dunn’s multiple comparisons correction, with two-tailed p values shown above linking lines when two-tailed p ≤ 0.05, and fold changes are shown between the columns.