Figure 3: Seizure Reduction by Seizure Onset Location and Other Characteristics –
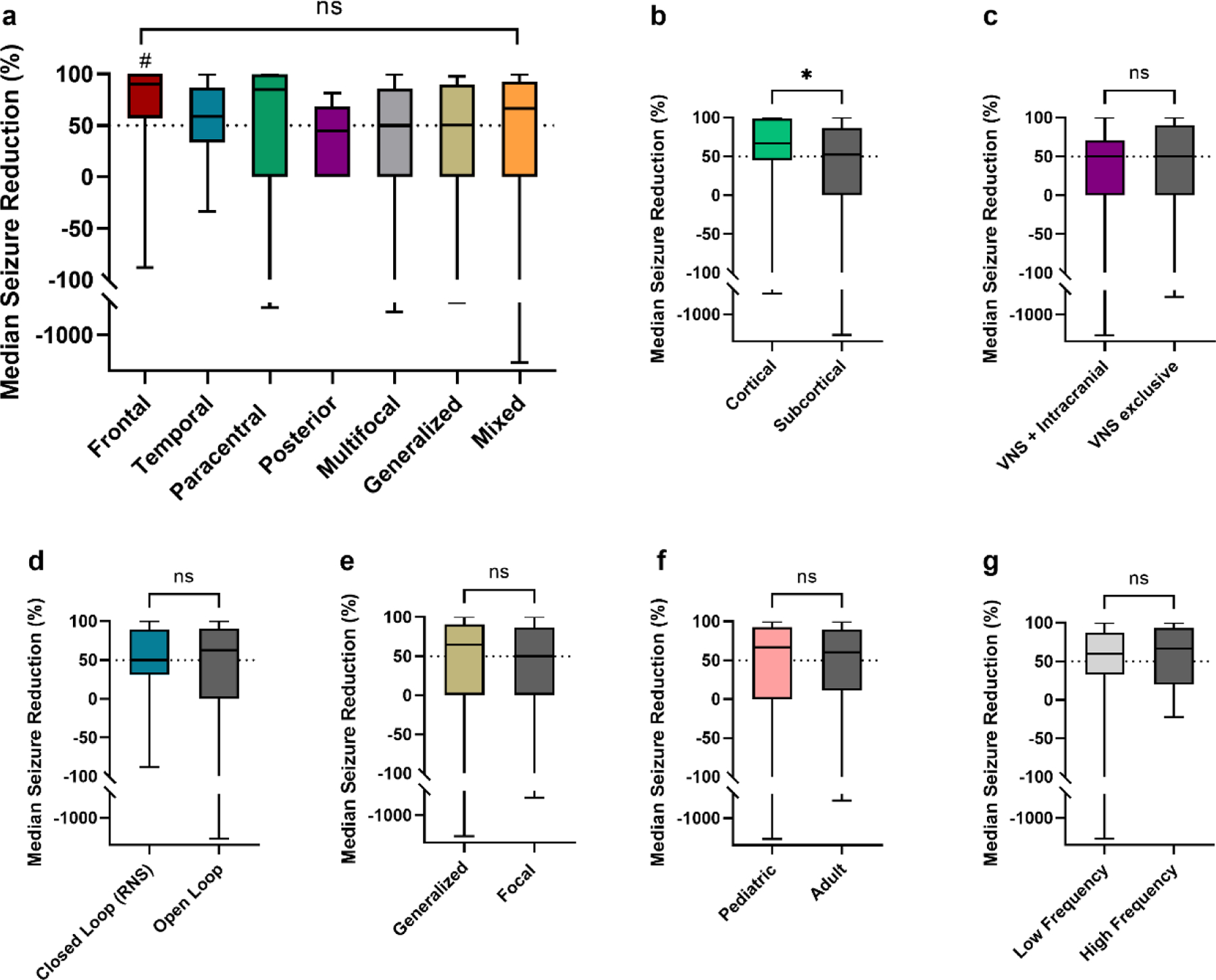
(a) MSR was not different across seizure onset groups. In pairwise comparions, frontal onset epilepsy patients (n=15) showed improved MSR compared to posterior (n=6) and multifocal patients (n=37) (# = p values < 0.05, unadjusted); but not compared to temporal (n=43), paracentral (n=20), generalized (n=15) and mixed (n=23) onsets. (b) Cortical stimulation (RNS and CSS, n=62) compared to subcortical stimulation (DBS and VNS, n=97) showed improved outcomes (* p = 0.03, unadjusted). (c) There was no difference in outcomes for active VNS device at the time of implantation with intracranial neurostimulation (DBS, RNS, CSS, n=34) vs. patients with only VNS stimulation (n=40). (d) There was no difference between closed loop stimulation (RNS) and open loop stimulation (DBS, CSS, VNS). (e) In patients implanted with subcortical stimulation devices (ANT-DBS, CM-DBS and VNS) seizure reduction was non significantly different in patients with generalized and mixed epilepsies (n=38) compared to focal and multifocal epilepsies (n=59). (f) There was no difference in MSR for pediatric patients (<18 years old at the time of implantation, n=37) vs. adults (≥18 years old at the time of implantation, n=122). (g) DBS, RNS and CSS patients with low frequency stimulation (<100 Hz) at last follow up (n=72) had comparable MSR than those with high frequency stimulation at last follow-up (n=47).
ANT-DBS, anterior thalamic nuclei deep brain stimulation; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; RNS, responsive neurostimulation; VNS, vagus nerve stimulation.
