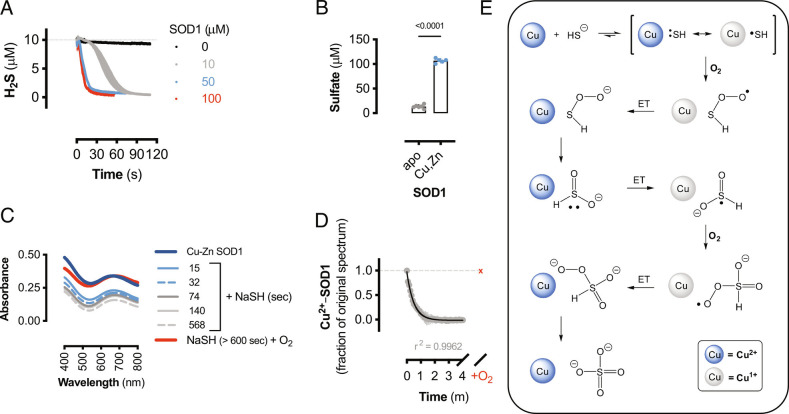
Fig. 2.
SOD1 is a sulfide oxidase. (A) Amperometric electrode measurements of H2S (10 μM) consumption by the addition of human SOD1 (10 to 100 μM) or buffer (0 μM) at t = 0. Data shown are mean values (±SEM, n = 3 reactions). (B) Sulfate measurements from 100 μM NaSH alone or reacted with 150 μM human Cu-Zn SOD1 or apo-SOD1. Data shown are mean values (±SEM, n = 5 to 6 reactions). Significance calculated by unpaired, two-tailed t test. (C) Visible spectra of bovine SOD1 alone (dark blue) and reacted with NaSH for indicated times under anaerobic conditions and after exposure to air (red line). (D) Change in absorbance (680 nm) from bovine SOD-Cu2+ reduction by NaSH as in (C). Gray circles represent anaerobic spectra, and red “x” represents air-treated samples. Data shown are mean values (±SEM; n = 3 reactions) and fit to a second-order decay curve (black line) with corresponding goodness of fit. (E) Proposed chemical mechanism for the oxidation of sulfide to sulfate by SOD1. Blue circles represent Cu2+-SOD1, gray circles represent Cu1+-SOD1, and ET represents electron transfer reaction.