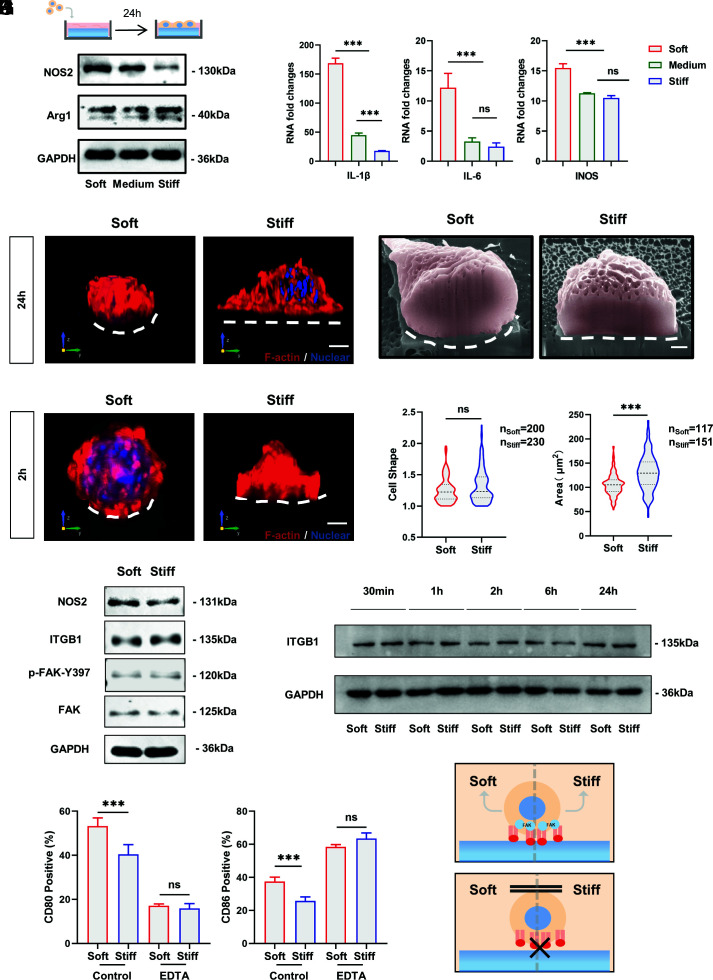
Fig. 2.
Adhesion is required for macrophage stiffness sensing, and macrophage inflammatory activation is independent of integrin-mediated FAK phosphorylation. (A and B) RAW264.7 cells were cultured on RGD-coated PVA hydrogel for 24 h using Western blotting and RT–PCR to detect the expression of inflammatory markers and factors. (C) Representative 3D rendering images of RAW264.7 cells cultured on soft and stiff PVA hydrogels for 24 h. F-actin is red, and nuclei are blue. (Scale bar, 3 μm.) (D) Representative FIB-SEM images of RAW264.7 cells after 24 h of culturing on soft and stiff PVA hydrogels. The white dashed line marks the ventral shape of the cell. (Scale bar, 1 µm.) (E) Representative 3D rendering images of RAW264.7 cells cultured on soft and stiff PVA hydrogels for 2 h. F-actin is red, and nuclei are blue. (Scale bar, 3 μm.) (F and G) Quantification of cell shape (ratio of maximum cell diameter to minimum cell diameter) and cell area on soft and stiff substrates. Data were analyzed using a nonparametric test. (H) To clarify whether macrophages show stiffness-related functional alterations at early stages and whether the classical pathway of integrins is involved, Western blotting was performed to examine protein expression after 2 h of culturing on hydrogels. (I) Expression of ITGB1 over time on substrates with different stiffnesses. (J) Inflammatory marker expression of RAW264.7 cells on substrates with different stiffnesses by flow cytometry after EDTA inhibition of integrin function (n = 3). (K) Schematic illustration. Macrophage stiffness sensing requires integrin-mediated adhesion. The data presented were analyzed by one-way ANOVA with Tukey’s posttest unless otherwise stated. *P < 0.033, **P < 0.002, ***P < 0.001; ns, no significant difference.