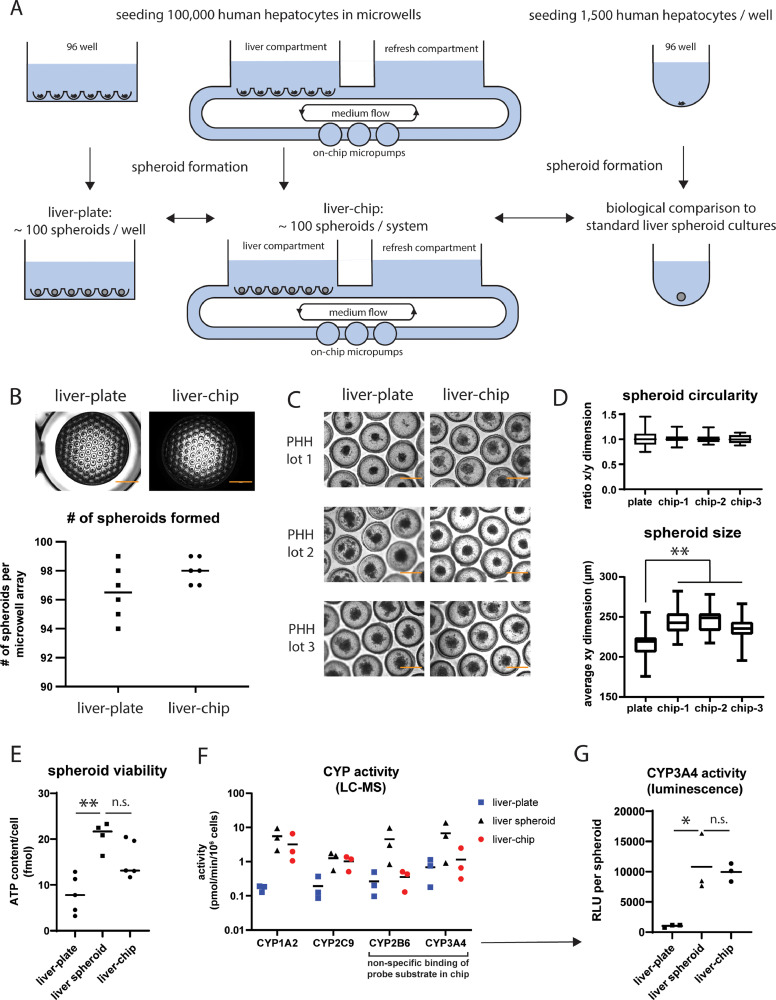
Fig. 2.
Human liver spheroid formation in a plate and organ-chip using microwells.
A: overview figure of the culture setup showing immediate seeding of PHH in microwells in a 96-well plate and organ-chip. Both models were biologically compared to conventional liver spheroid cultures, formed by seeding cells in low-attachment wells. B: brightfield pictures showing spheroids formed in a microwell array in a plate and organ-chip after 7 days of culture. The number of spheroids per system was shown in the graph below, which represents the mean + individual values. N = 6 individual cultures. Scale bar, 2000 µm. C: brightfield pictures of spheroids formed after 6-10 days of culture in microwells in a plate and organ-chip. Spheroid formation was successful in all three tested PHH lots. Scale bar, 400 µm. D: spheroid size and circularity were quantified in fully formed spheroid cultures (day 7-8 of culture) by measuring the x- and y-dimensions of spheroids in individual microwell array in both the plate and organ-chip. Each box plot summarizes data from 21-27 individual spheroids within the same microwell array. **, p < 0.01. E: spheroid viability of the liver-plate, liver-chip and liver spheroid culture after 2 weeks of culture. The graph shows the mean and individual values expressed as fmol ATP/cell. **, p < 0.01; n.s., not significant (p ≥ 0.05). F: CYP enzyme activity (determined using LC-MS quantification of primary metabolites) in liver-chip, liver-plate, and liver spheroids after 2 weeks of culture. The graph shows the mean and individual values obtained from 3 different donors. G: CYP3A4 activity per spheroid (as measured by a luminescence-based assay) of the liver-plate, liver-chip and single liver spheroid after 2 weeks of culture. The graph shows the mean and individual values per condition. The luminescent signal is expressed in relative luminescent units (RLU) and corrected for medium volume and number of spheroids per condition. *, p < 0.05; n.s., not significant (p ≥ 0.05).