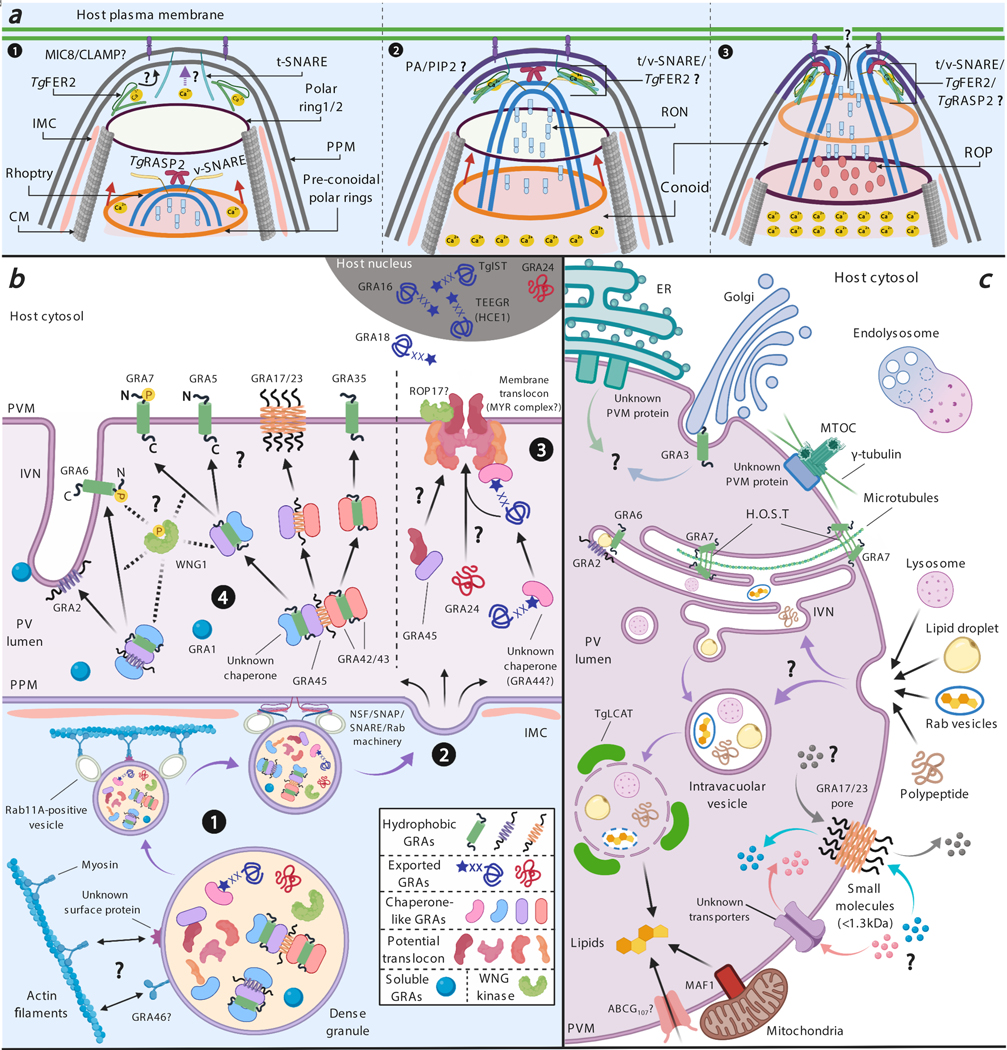
Figure 2. (A) Model for rhoptry secretion.
1) The process of host cell invasion raises the parasite internal [Ca2+] and the upper polar ring and conoid are extended until the base of the conoid protrudes beyond the lower polar ring (62). MICs are secreted, and MIC8/CLAMP could bind to the parasite and host plasma membrane (67, 120). The increase in [Ca2+] or the presence of MICs on the PPM could trigger a signaling cascade that changes the parasite phospholipid composition. The retracted conoid with rhoptry organelle moves through the cortical microtubules towards the apical polar ring. TgRASP and a putative v-SNARE protein are present at the membrane of the apical end of the rhoptry. A putative t-SNARE and TgFER2 at the PPM could bind to the membrane upon sensing a calcium signal. 2) When the conoid and the apical end of the rhoptry (containing RON proteins) are close to the apical polar ring, TgFER2 could mediate the interaction of the t/v-SNARE and bring the rhoptry membrane closer to the PPM, which could allow TgRASP2 to interact with PA/PIP2 (Phosphatidic Acid/Phosphatidylinositol (4,5)-bisphosphate) on the PPM. 3) The conoid ultimately passes through the apical polar ring, and the t/v-SNARE/TgFER2/TgRASP2 complex could mediate the fusion of the rhoptry membrane and the PPM allowing RONs/ROPs to be secreted. Possibly MIC/ROP/RON secretion changes the permeability of the host plasma membrane at the attachment site allowing RONs and ROPs to pass through and reach the cytosol. (B) Model for trafficking of GRAs from dense granules to their destinations. (1) Inside the dense granules, hydrophobic GRAs are packed as soluble oligomers via shielding of their hydrophobic domains by putative chaperone-like GRAs to avoid fusion with the dense granule membrane. Mature dense granules bind to myosin and/or Rab11A-positive vesicles, which mediate the transport of dense granules via actin tracks to the parasite’s periphery where they dock at IMC gaps. (2) Docking of dense granules at an IMC gap allows their fusion with the PPM via the NSF/SNAP/SNARE/Rab machinery resulting in exocytosis of GRAs into the PV lumen. (3) Once secreted into the PV lumen, ASP5-processed TEXEL-containing exported GRAs with potential N-terminal acylation are escorted by unknown chaperone GRAs to the PVM translocon, of which MYR1/2/3/4 and GRA44 may be components. GRA45 likely functions as a chaperone helping the translocon components traffic to and insert into the PVM. GRA24 is a TEXEL-negative exported protein and traffics to the membrane translocon via an unknown mechanism followed by translocation into the host cell. (4) Hydrophobic GRAs (transmembrane, amphipathic helices or α-helices) are escorted by chaperone GRAs to traffic inside the PV lumen and reach their destinations through an unknown mechanism. WNG kinase (WNG1) is involved in the eventual insertion of GRAs into the PVM or IVN via either directly phosphorylating the hydrophobic GRAs or phosphorylating the chaperone GRAs leading to the dissociation of hydrophobic GRA cargo from the chaperone. (C) Nutrient and small molecule import across the PVM. Host organelles, such as endoplasmic reticulum (ER), Golgi apparatus and the microtubule organizing center (MTOC), are recruited to the PVM. The relocation of the MTOC leads to the conversion of the microtubules network around the PVM bringing vesicular organelles, such as endolysosomal vesicles, to its vicinity where they can be used as nutrient resources for Toxoplasma. Host Golgi is retained in PVM invaginations by GRA3 whereas ER and MTOC are recruited via unknown PVM proteins. Host microtubules are recruited to invaginations of the PVM, mediated by GRA7, where they form a structure named H.O.S.T. responsible for cholesterol uptake derived from host lysosomes. Small nutrients are likely acquired through a membrane pore formed by GRA17/23 and/or other unknown PVM transporters. Host lysosomes, lipid droplets, Rab-positive vesicles derived from the endolysosomal system as well as polypeptides are invaginated into the PV and transiently stored at the IVN and intravacuolar vesicles of which the outer membrane can be digested by TgLCAT resulting in the release of nutrients into the PV lumen. GRA2 and GRA6 are present in the IVN and mediate the uptake of host cytosolic proteins. Host mitochondria are anchored to the PVM by MAF1. ABCG107 is a potential PVM transporter for scavenging host lipids and maintaining lipid homeostasis inside the PV lumen.