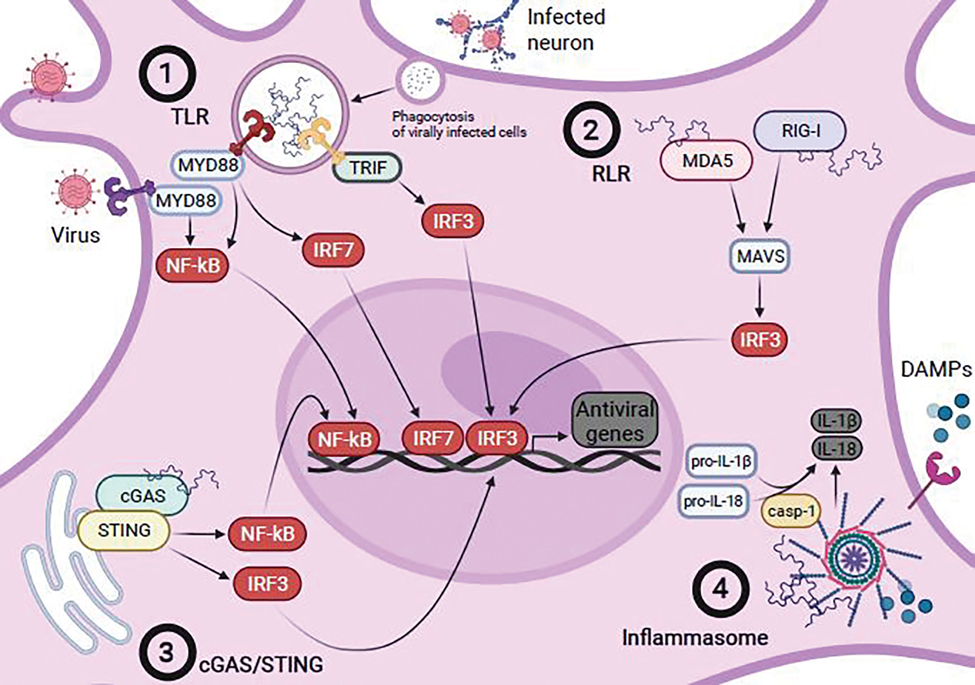
Figure 1.
Proposed mechanisms of microglial recognition of viral infection. (1) TLR recognition of viral DNA or RNA, either through direct sensing of virus or following phagocytosis of virally-infected cells, leads to the recruitment and activation of downstream modulators such as MyD88 or TRIF. MyD88-dependent signaling leads to the activation and nuclear localization of the major mediator of inflammatory responses, NFκB, or interferon regulatory factor 7 (IRF7), while activation of TRIF leads to activation and nuclear localization of another critical mediator of innate immune signaling, interferon regulatory factor 3 (IRF3). (2) Recognition of cytoplasmic viral RNA by RLRs RIG-I or MDA5 results in activation of downstream mediator MAVS, which in turn leads to activation and nuclear localization of IRF3. (3) cGAS serves recognizes cytoplasmic viral nucleic acids, leading toSTING activation which in turn activates both NFκB and IRF3, leading to their nuclear localization. (4) Viral components and DAMPs released by transduced or damaged cells activate the inflammasome, leading to the recruitment and activation of caspase-1 (casp-1), which cleaves pro-IL-1β and pro-IL-18 to their active forms, IL-1β and IL-18. Microglia likely integrate these signaling pathways following recognition of viral infection directly or by virally-induced signals from other cells to produce a wide array of antiviral genes such as inflammatory cytokines and chemokines that both serve to clear virus and coordinate the antiviral responses of other cells.