Abstract
Introduction:
There is a critical need to accurately stratify liver transplant (LT) candidates’ risk of post-LT mortality prior to LT to optimize patient selection and avoid futility. Here, we compare previously described pre-LT clinical risk scores with the recently developed Liver Immune Frailty Index (LIFI) for prediction of post-LT mortality. LIFI measures immune dysregulation based on pre-LT plasma HCV IgG, MMP3 and Fractalkine. LIFI accurately predicts post-LT mortality, with LIFI-low corresponding to 1.4% 1-year post-LT mortality compared with 58.3% for LIFI-high (C-statistic=0.85).
Methods:
LIFI was compared to MELD, MELD-Na, MELD 3.0, D-MELD, MELD-GRAIL, MELD-GRAIL-Na, UCLA-FRS, BAR, SOFT, P-SOFT, and LDRI scores on 289 LT recipients based on waitlist data at the time of LT. Survival, hazard of early post-LT death, and discrimination power (C-statistic) were assessed.
Results:
LIFI showed superior discrimination (highest C-statistic) for post-LT mortality when compared to all other risk scores, irrespective of biologic MELD. On univariate analysis, the LIFI showed a significant correlation with mortality 6-months, as well as 1-, 3-, and 5-years. No other pre-LT scoring system significantly correlated with post-LT mortality. On bivariate adjusted analysis, African American race (p<0.05) and pre-LT cardiovascular disease (p=0.053) were associated with early- and long-term post-LT mortality. Patients who died within 1-yr following LT had a significantly higher incidence of infections, including 30-day and 90-day incidence of any infection, pneumonia, abdominal infections, and UTI (p<0.05).
Conclusions:
LIFI, which measures pre-LT biomarkers of immune dysfunction, more accurately predicts risk of post-LT futility compared with current clinical predictive models. Pre-LT assessment of immune dysregulation may be critical in predicting mortality after LT and may optimize selection of candidates with lowest risk of futile outcomes.
Keywords: Liver Transplant, Cirrhosis, Pre-Transplant Risk Assessment, Liver Transplant Futility, Clinical Risk Score, Post-Transplant Mortality
Contribution To The Field
End-stage liver disease (ESLD) is the 11th leading cause of death in the US. The only curative treatment available is liver transplantation (LT). Currently, demand exceeds donor availability, and one-third of waitlisted patients die awaiting LT. Based on the principles of justice, the “Final Rule” relies upon transplanting the sickest people while avoiding transplant futility. Multiple clinical risk predictors have been developed; however, they lack good discrimination (C-statistic > 0.70) or require incorporating donor and intra-operative information, limiting their utility prior to donor selection. In addition, none of these scores integrate transplant candidate immune dysfunction, which appears to be a critical component of overall patient severity of illness and susceptibility to post-LT complications. We recently introduced a novel pre-LT risk score, the Liver Immune Frailty Index (LIFI), based on pre-transplant biomarkers of immune dysfunction, which showed excellent discrimination of post-LT one-year mortality. In the current study, we compared the discrimination power of LIFI for predicting one-year post-LT mortality to currently utilized risk scores. Herein, we showed that LIFI had a superior discrimination (C-statistic 0.85) to predict one-year post-LT mortality. Immune function assessment before LT may predict mortality after LT and optimize the current candidacy with the lowest risk of futile outcomes.
Introduction
Liver transplant (LT) is the only curative treatment for end-stage liver disease (ESLD); however, due to the current shortage of donor organs, as many as one third of patients on the waiting list will expire awaiting LT (1). The current organ allocation model relies on the “Final Rule,” based on the principle of transplanting the “sickest first” while avoiding LT in patients at risk for futile outcomes (2,3). To accomplish this, patients are risk-stratified by using the Model for End Stage Liver Disease (MELD) (4,5). Despite its clinical utility in estimating risk of wait-list mortality, MELD fails to accurately predict the risk of death following LT (6). Additional scoring systems have been proposed to better risk-stratify patients.
MELD was initially developed to assess mortality in cirrhotic patients undergoing transjugular intrahepatic portosystemic shunts (TIPS) (7). Subsequently, MELD has been validated to predict three-month waitlist mortality in candidates awaiting LT (5). For the purpose of waitlist stratification, MELD was later updated to include sodium, which is an independent risk factor of waitlist mortality, resulting in current use of MELD-Na as the pre-LT acuity score (8). Several variations of MELD have been proposed to increase sensitivity for severe disease, including the addition of variable such as female sex and albumin level to account for allocation inequities. To capture renal function more accurately in the cirrhotic patient, MELD 3.0 (9) and GFR assessment of liver disease (GRAIL) scores (MELD-GRAIL and MELD-GRAIL Na) were developed (10). Incorporating these additional components increases sensitivity for 90-day waitlist mortality, especially in decompensated cirrhotics and women (11). MELD variations were developed for assessment of pre-LT mortality, but application to predict post-transplant outcomes have been deficient.
Additional scores have been proposed specifically for pre-LT risk assessment of post-LT mortality. These commonly incorporate recipient co-morbidities in addition to donor factors. The D-MELD combines the donor’s age and the recipient’s preoperative calculated MELD score to predict survival and length of hospitalization (LOS) after LT (12). The Balance of Risk (BAR) score is a simplified score that includes recipient age, MELD, life-support requirement, and retransplant in addition to donor age and cold ischemia time (13). More complex scores, such as the Survival Outcomes Following liver Transplantation (SOFT) score, utilize 18 donor, recipient, and graft variables (14). A variation including only pre-procurement variables (P-SOFT) can risk stratify waitlisted patients (14). The Liver Donor Risk Index (LDRI) determines risk of post-liver transplant graft failure based on seven donor and graft characteristics (15). Finally, the UCLA Futility Risk Score (UCLA-FRS) further discriminates risk of post-LT futility in patients with MELD scores ≥40, using the age adjusted Charlson Comorbidity Index (CCI) (16), MELD, sepsis within 30 days, and cardiac risk (17).
Of these risk stratification tools, BAR and SOFT have the highest previously described discrimination for pre-LT prediction of post-LT mortality; however, none have a concordance (C-statistic >0.7) (8–15,17,18). In addition, many incorporate donor and intraoperative information, which limits their utility prior to donor selection (13,14). Ultimately, recipient candidacy relies heavily on subjective clinical judgment and is prone to selection bias (19). Objective parameters to accurately stratify patients’ risk of post-LT death prior to LT are lacking. This, in conjunction with increasing recipient severity of illness at the time of transplant (17,20–23), risks futile LT. There is a critical need to accurately stratify LT candidates’ risk of post-LT mortality prior to LT to optimize patient selection and avoid futility.
Transplant candidate immune dysfunction appears to be a critical component of overall patient severity of illness and susceptibility to complications. Cirrhosis ultimately leads to profound metabolic and immunologic dysfunction (24,25), resulting in both physical and immunologic frailty (23). Despite a clear association with post-transplant outcomes, immune dysfunction is not currently utilized in pre-LT candidate assessment. We have recently introduced a novel pre-LT risk score, the Liver Immune Frailty Index (LIFI), based on pre-transplant biomarkers of immune dysfunction. Using weighted points assigned to pre-LT HCV-IgG status and plasma concentrations of Fractalkine and MMP3, LIFI stratifies patients into high-, moderate-, and low-risk of early post-LT mortality (death ≤1yr). On internal validation, LIFI predicts futility with a C-statistic=0.84, which exceeds predictive capacity of previously described models. Specifically, LIFI-high LT recipients had a 58.3% risk of 1-year mortality post-LT compared to 12.7% for LIFI-moderate and 1.4% for LIFI-low (23).
Here, we compare the discrimination power of LIFI for predicting 1-year and long-term post-LT mortality to currently utilized risk scores for LT candidate assessment in the same patient cohort. Outcomes are further stratified by recipient MELD score <30 vs ≥30, as recent studies show MELD>30 is an independent risk factor for poor clinical outcomes following LT (26,27).
Methods
2.1. Study cohort
Adult patients on the waitlist for liver transplant at Houston Methodist Hospital (HMH, January 1, 2013 – December 31, 2017) and University Hospital/Rutgers New Jersey Medical School (Rutgers NJMS, January 1, 2018 – December 31, 2021) were evaluated for inclusion. All protocols were approved by each institution’s respective institutional review board (IRB) and human subject research was conducted in accordance with the ethical principles of the Declaration of Helsinki (28).
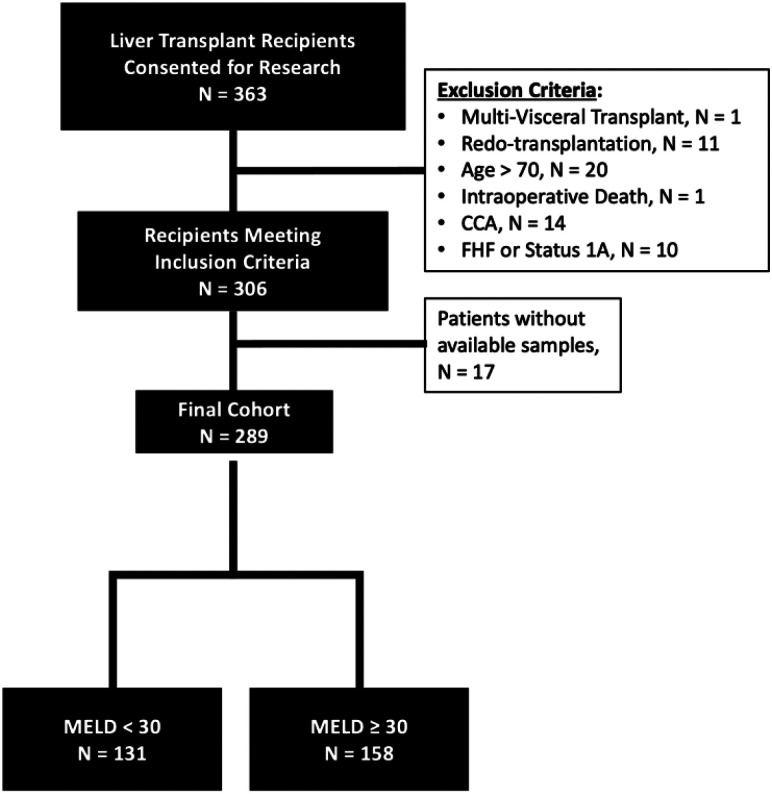
Adult recipients (age ≥18 years old) who received a deceased donor whole LT with at least 12 months of follow-up were considered for analysis. Patients with a history of cholangiocarcinoma, fulminant hepatic failure, expiring during the transplant procedure, or receiving multi-visceral transplants other than liver-kidney were excluded. A total of 289 patients were included in the final analysis (Figure 1).
Figure 1.
Flowchart diagram of inclusion and exclusion criteria.
2.2. Data collection
Pre-LT recipient demographic data (age, sex, race, weight, height, BMI, primary cause of end-stage liver disease [ESLD]) and comorbidities (liver cold ischemia time, prior abdominal surgery, chronic renal insufficiency [CRI], diabetes mellitus, cardiac comorbidities [defined as prior MI, stent, valvular insufficiency, coronary artery stenosis >70%, and arrythmia], peptic ulcer disease, chronic pulmonary disease, cancer, and clinical findings of portal hypertension [ascites, encephalopathy, variceal bleeding]) were documented. Pre-LT medical acuity was recorded, including MELD or MELD-Na, vasopressors, ventilator, dialysis, hospital length-of-stay (LOS), and intensive care unit (ICU) LOS. Pre-LT infections (defined as those occurring <30 days prior to LT) were assessed, including pneumonia, peritonitis, sepsis, and urinary tract infection (UTI) based on the occurrence of positive culture data. Donor demographics include age, cause of death, terminal creatinine, deceased donor type (brain death or circulatory death), and location. Recipient laboratory data (albumin, sodium, creatinine, total bilirubin, AST, ALT, INR, hemoglobin, platelet count, and white blood cell count with differential) were obtained at the time of transplant. Both calculated biologic laboratory and list MELD scores were included, as reported to the United Network of Organ Sharing (UNOS). The MELD-Na was utilized for all patients following policy induction after January 2016 (29).
The primary outcome was patient survival, assessed at 6-months, as well as 1-, 3-, and 5-years post-LT. Secondary outcomes included post-LT complications, including death, hospital length of stay, ICU length of stay, presence of cardiac morbidity after LT, and infectious complications within 30 and 90-days after LT. Severe infection was defined as sepsis, pneumonia, or intra-abdominal infection within 90 days following LT.
2.3. Score model calculations
The LIFI score was calculated as previously described (23). LIFI was compared to currently accepted medical acuity scoring systems suggested for the liver transplant population. Specifically, the MELD, MELD-Na, MELD 3.0, D-MELD, MELD-Grail, Meld Grail-Na, UCLA-FRS, BAR, SOFT, P-SOFT, and LDRI (5,8,9,11–15,17). Formulas used to calculate each score and their distribution can be found in Supplemental Table 1.
2.4. Statistics
Continuous variables are reported as median and interquartile range (IQR) and categorical variables as frequencies and proportions. Differences between groups were compared using the ANOVA or the Kruskal Wallis test for continuous variables and the Chi-square or Fisher’s exact tests for categorical variables, as appropriate. Bivariate analysis was used to evaluate patient characteristics, stratified by post-transplant mortality at 3- and 6-months, as well as 1-, 3-, and 5-years. Univariate Cox regression modeling was used to determine the hazard ratio for post-transplant mortality at 3- and 6-months, as well as 1-, 3-, and 5-years of individual risk scores and their components. The discrimination power of individual risk scores was determined by the C-statistic and compared with that of the LIFI score using the chi-square test. All analyses were performed on Stata version 17.0 (StataCorp LLC, College Station, TX, USA). A p-value of <0.05 was considered statistically significant.
Results
3.1. Comparison of donor and recipient demographics.
A total of 289 patients receiving LTs between January 2013-December 2021 and were included in final analysis (Figure 1) and overall patient demographics are shown in Table 1. LT recipient outcomes were compared for patient who survived ≥1 year versus those who expired <1 year following LT (Table 1). Black race (p = 0.02), history of cardiovascular disease (p = 0.05), and intraabdominal infection within 30 days of LT (p = 0.01) were all associated with 1-year post-LT mortality (Table 1). On bivariate analysis based on time to mortality, only pre-LT cardiac morbidity was significantly associated with early mortality at 6-months and 1-year (p < 0.05). Long-term mortality (3- and 5-year) was associated with older age at LT (p <0.05) (Supplemental Table 2).
Table 1.
Study population characteristics
| Total (N=289) | Alive (n=272) | Dead (n=17) | p-value | |
|---|---|---|---|---|
| Recipient Demographics | ||||
| Age at OLT, median (IQR) | 56.6 (48.2, 62.5) | 56.4 (47.5, 62.4) | 59.8 (56.6, 63.9) | 0.06 |
| Male gender | 197 (68.2) | 185 (68.0) | 12 (70.6) | 0.83 |
| Recipient race | ||||
| Caucasian | 177 (61.2) | 168 (61.8) | 9 (52.9) | 0.13 |
| African American | 33 (11.4) | 28 (10.3) | 5 (29.4) | |
| Hispanic | 62 (21.5) | 60 (22.1) | 2 (11.8) | |
| Asian | 8 (2.8) | 8 (2.9) | 0 (0.0) | |
| Other | 9 (3.1) | 8 (2.9) | 1 (5.9) | |
| Recipient race - Black | 33 (11.4) | 28 (10.3) | 5 (29.4) | 0.02 |
| SKLT | 35 (12.1) | 32 (11.8) | 3 (17.6) | 0.47 |
| 28.5 (24.8, 32.6) | 28.6 (24.9, 32.8) | 25.4 (23.0, 32.2) | 0.14 | |
| BMI (kg/m2), median (IQR) | ||||
| Cause of ESLD Code | ||||
| HCV | 80 (27.7) | 69 (25.4) | 11 (64.7) | 0.13 |
| EtOH | 110 (38.1) | 108 (39.7) | 2 (11.8) | |
| NASH/Cryptogenic | 57 (19.7) | 53 (19.5) | 4 (23.5) | |
| Other | 42 | 42 | 15.3 | |
| CRI | 72 (25.0) | 67 (24.7) | 5 (29.4) | 0.66 |
| Diabetes | 97 (33.6) | 94 (34.6) | 3 (17.6) | 0.15 |
| Cardiovascular | 22 (7.6) | 8 (2.9) | 2 (11.8) | 0.05 |
| Pre-transplant Labs, median (IQR) | ||||
| T. bilirubin (mg/dL) | 8.7 (1.5, 22.9) | 8.8 (1.5, 23.1) | 5.3 (2.1, 19.0) | 0.39 |
| INR | 2.0 (1.4, 2.5) | 2.0 (1.4, 2.5) | 2.3 (1.4, 3.2) | 0.61 |
| BUN (mg/dL) | 28.4 (22.0, 42.0) | 28.3 (21.8, 42.2) | 30.3 (24.8, 39.4) | 0.65 |
| Creatinine (mg/dL) | 1.1 (0.8, 1.8) | 1.1 (0.8, 1.8) | 1.1 (1.0, 2.0) | 0.62 |
| Albumin (g/L) | 3.1 (2.7, 3.6) | 3.2 (2.7, 3.7) | 2.9 (2.7, 3.6) | 0.50 |
| Sodium (mEq/L) | 139.0 (136.0, 141.0) | 139.0 (136.0, 141.0) | 141.0 (139.0, 141.0) | 0.23 |
| Pre-transplant Medical Acuity | ||||
| Pressors | 62 (21.5) | 56 (20.6) | 6 (35.3) | 0.15 |
| Vent | 80 (27.7) | 72 (26.5) | 8 (47.1) | 0.07 |
| LOS (days), median (IQR) | 7.0 (1.0, 17.0) | 7.0 (1.0, 17.0) | 7.0 (1.0, 23.0) | 0.83 |
| ICU LOS (days), median (IQR) | 0.0 (0.0, 7.0) | 0.0 (0.0, 7.0) | 6.0 (0.0, 13.0) | 0.30 |
| RRT | 126 (43.6) | 117 (43.0) | 9 (52.9) | 0.42 |
| Pre-transplant Infections (30 days prior to LT) | ||||
| Any Positive Cultures | 104 (36.0) | 98 (36.0) | 6 (35.3) | 0.95 |
| Sepsis | 32 (11.1) | 30 (11.0) | 2 (11.8) | 0.93 |
| Pneumonia | 39 (13.5) | 37 (13.6) | 2 (11.8) | 0.83 |
| UTI | 57 (19.7) | 54 (19.9) | 3 (17.6) | 0.82 |
| Intraabdominal Infection | 21 (7.3) | 17 (6.3) | 4 (23.5) | 0.01 |
IQR, interquartile range; OLT, orthotopic liver transplantation; SKLT, Simultaneous Liver Kidney Transplant; BMI, body mass index; ESLD, end-stage liver disease; HCV, hepatitis C; EtOH, alcohol abuse disorder; NASH, non-alcoholic steatohepatitis; INR, international normalized ratio; BUN, blood urea nitrogen; CRI, chronic renal insufficiency; LOS, length of hospitalization; ICU, intensive care unit; UTI, urinary tract infection.
3.2. MELD Score Correlates with Pre-LT Patient Severity of Illness but Fails to Discriminate Patient Mortality.
Patients were next stratified into subgroups based on laboratory MELD as reported to UNOS at LT (MELD≥30, n= 158 and MELD<30, n=131). Overall study population and subgroup patient characteristics are shown in Table 2. Compared to MELD<30, recipients with MELD≥30 were older (p < 0.01), had higher BMI (p < 0.001), less commonly had HCC exception at time of LT (p < 0.001), and varied with regards to liver disease etiology (p < 0.01, Table 2). As expected, chronic renal insufficiency (CRI) was more common in MELD ≥30 (p = 0.01). The higher MELD cohort also had more frequent pre-LT incidence of peptic ulcer disease (PUD), encephalopathy at LT, pre-LT infections, ICU-level care, ventilator use, vasopressor use, and need for dialysis (all p<0.05). Finally, recipients with MELD≥30 received grafts from younger, locally located, and donation after brain death (DBD) donors, (all p < 0.05, Table 2).
Table 2.
Study population, stratified by MELD
| Recipient Demographics | ||||
| Age at OLT, median (IQR) | 56.6 (48.2, 62.5) | 58.3 (52.5, 63.4) | 54.0 (45.6, 60.7) | <0.001 |
| Male gender | 197 (68.2) | 92 (70.2) | 105 (66.5) | 0.49 |
| Other | 9 (3.1) | 6 (4.6) | 3 (1.9) | |
| SKLT | 35 (12.1) | 13 (9.9) | 22 (13.9) | 0.30 |
| BMI (kg/m2), median (IQR) | 28.5 (24.8, 32.6) | 27.1 (24.1, 30.5) | 29.8 (25.5, 34.4) | <0.001 |
| HCC Exception | 68 (23.5) | 59 (45.0) | 9 (5.7) | < 0.001 |
| Other | 7 (2.4) | 4 (3.1) | 3 (1.9) | |
| CRI | 72 (25.0) | 23 (17.6) | 49 (31.2) | 0.01 |
| Diabetes | 97 (33.6) | 46 (35.1) | 51 (32.3) | 0.61 |
| CAD | 22 (7.6) | 8 (2.9) | 2 (11.8) | 0.05 |
| PVD | 6 (2.1) | 1 (0.8) | 5 (3.2) | 0.15 |
| COPD | 19 (6.6) | 6 (4.6) | 13 (8.2) | 0.21 |
| Connective Tissue Disease | 6 (2.1) | 1 (0.8) | 5 (3.2) | 0.15 |
| PUD | 23 (8.0) | 5 (3.8) | 18 (11.4) | 0.02 |
| Encephalopathy | <0.001 | |||
| None | 98 (33.9) | 72 (55.0) | 26 (16.5) | |
| Grade 1–2 | 144 (49.8) | 53 (40.5) | 91 (57.6) | |
| Grade 3–4 | 47 (16.3) | 6 (4.6) | 41 (25.9) | |
| Pre-transplant Labs, median (IQR) | ||||
| T. bilirubin (mg/dL) | 8.7 (1.5, 22.9) | 1.4 (0.7, 3.5) | 19.8 (10.3, 29.9) | <0.001 |
| INR | 2.0 (1.4, 2.5) | 1.3 (1.1, 1.7) | 2.4 (2.0, 2.9) | <0.001 |
| BUN (mg/dL) | 28.4 (22.0, 42.0) | 26.2 (19.2, 32.5) | 32.1 (24.7, 53.6) | <0.001 |
| Creatinine (mg/dL) | 1.1 (0.8, 1.8) | 0.9 (0.7, 1.3) | 1.4 (1.0, 2.3) | <0.001 |
| Albumin (g/L) | 3.1 (2.7, 3.6) | 3.3 (2.7, 3.7) | 3.0 (2.7, 3.6) | 0.31 |
| Sodium (mEq/L) | 139.0 (136.0, 141.0) | 139.0 (136.0, 141.0) | 139.0 (136.0, 141.0) | 0.43 |
| Pre-transplant Medical Acuity | ||||
| Vasopressors Use at LT | 62 (21.5) | 7 (5.3) | 55 (34.8) | <0.001 |
| Ventilator Use at LT | 80 (27.7) | 8 (6.1) | 72 (45.6) | <0.001 |
| LOS (days), median (IQR) | 7.0 (1.0, 17.0) | 1.0 (0.0, 1.0) | 15.0 (9.0, 23.0) | <0.001 |
| ICU LOS (days), median (IQR) | 0.0 (0.0, 7.0) | 0.0 (0.0, 0.0) | 6.0 (0.0, 16.0) | <0.001 |
| Dialysis | 126 (43.6) | 20 (15.3) | 106 (67.1) | <0.001 |
| Pre-transplant Infections (30 days prior to LT) | ||||
| Any Positive Cultures | 104 (36.0) | 27 (20.6) | 77 (48.7) | <0.001 |
| Sepsis | 32 (11.1) | 3 (2.3) | 29 (18.4) | <0.001 |
| Pneumonia | 39 (13.5) | 7 (5.3) | 32 (20.3) | <0.001 |
| UTI | 57 (19.7) | 18 (13.7) | 39 (24.7) | 0.02 |
| Intraabdominal Infection | 21 (7.3) | 4 (3.1) | 17 (10.8) | 0.01 |
| Donor Characteristics | ||||
| Donor Age | 31.0 (24.0, 45.0) | 36.0 (26.0, 50.0) | 29.5 (22.0, 39.0) | <0.001 |
| National | 12 (4.2) | 4 (3.1) | 8 (5.1) | |
| DCD Donor | 13 (4.5) | 10 (7.6) | 3 (1.9) | 0.03 |
IQR, interquartile range; OLT, orthotopic liver transplantation; SKLT, Simultaneous Liver Kidney Transplant; BMI, body mass index; HCC, hepatocellular carcinoma; ESLD, end-stage liver disease; HCV, hepatitis C; EtOH, alcohol abuse disorder; NASH, non-alcoholic steatohepatitis; CAD, coronary artery disease; PVD, peripheral vascular disease; COPD, chronic pulmonary disease; PUD, peptic ulcer disease; INR, international normalized ratio; BUN, blood urea nitrogen; CRI, chronic renal insufficiency; LOS, length of hospitalization; ICU, intensive care unit; UTI, urinary tract infection; DCD, donation after cardiac death.
Following transplant, recipients with MELD ≥30 experienced more frequent blood transfusions (p = 0.01) and had longer overall LOS (p < 0.001) as well as ICU LOS (p < 0.001). The incidence of post-transplant infections, including any positive cultures, documentation of sepsis, pneumonia, UTI, and intra-abdominal infection within 30- and 90-days were also significantly higher in MELD≥30 vs MELD <30 (p < 0.05). Despite the overwhelming differences in pre-and early post-transplant morbidity, there was no statistically significant difference in overall patient survival at 5 years (p = 0.95).
3.3. Clinical Risk Scores Correlate with Medical Acuity and MELD but Fail to Predict Mortality.
Previously described risk scores were calculated for all patients and compared between the MELD<30 and the MELD≥30 cohort (Supplemental Table 1). As expected, based on calculation parameters, the MELD, MELD-Na, MELD 3.0, D-MELD, MELD-GRAIL, MELD-GRAIL-Na, UCLA FRS, BAR, SOFT and P-SOFT were significantly higher in the high-MELD group (Table 4). The LDRI was the only score with significantly lower median values in the MELD≥30 group (66.4%) compared to MELD<30 (p <0.001) (Table 4). These results highlight that the aggregate LIFI score assesses parameters of recipient risk which are distinct from those evaluated by standard clinical risk scores.
Table 4.
Clinical risk assessment scores for overall population, and stratified by MELD (median, IQR)
| (N=289) | (n=131) | (n=158) | ||
|---|---|---|---|---|
| 1 (0.3) | 0 (0.0) | 1 (0.6) | ||
| 7 (2.4) | 0 (0.0) | 7 (4.4) | ||
| High (≥80) | 116 (40.1) | 18 (13.7) | 98 (62.0) | <0.001 |
IQR, interquartile range; MELD, Model for End-Stage Liver Disease; GRAIL, GFR assessment in liver disease; UCLA-FRS, University of California Los Angeles Futility Risk Score; BAR, balance of risk; SOFT, survival outcomes following liver transplantation; P-SOFT, predicted survival outcomes following liver transplantation;
3.4. LIFI has superior discrimination compared to all other clinical risk scores in both early and long-term post-LT mortality.
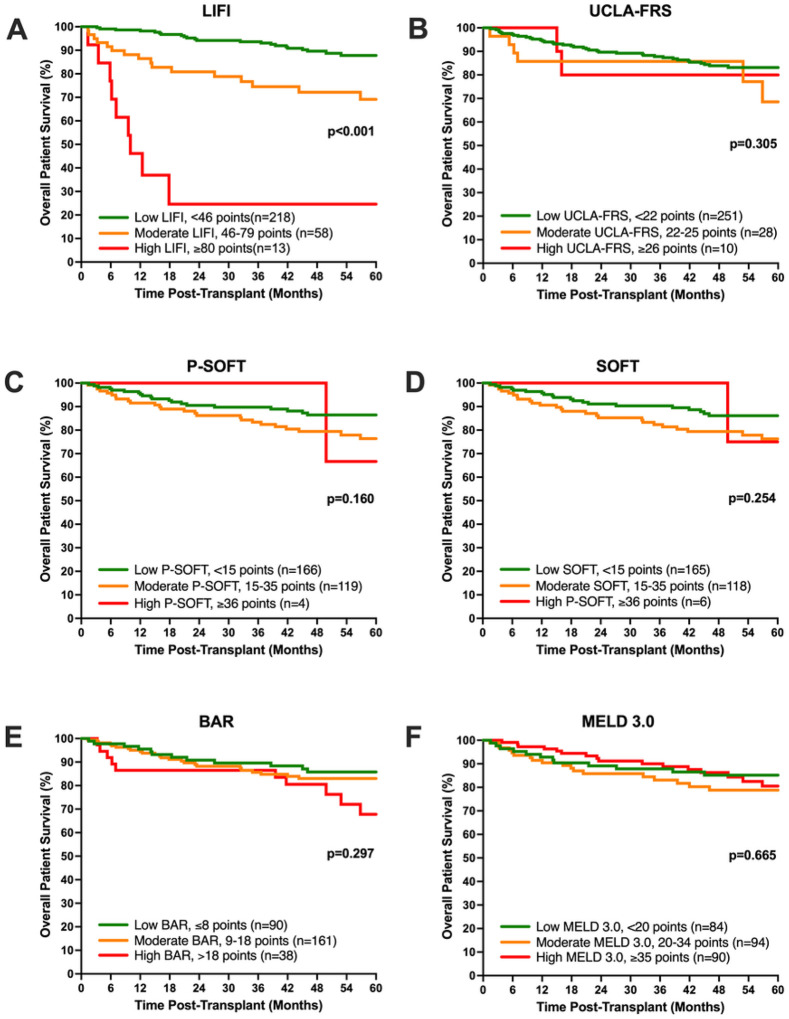
On univariable Cox proportional hazard analysis, the LIFI score was the only pre-transplant risk score with a significant correlation with early post-LT mortality at 6-months (HR 1.04 [1.03, 1.06] p < 0.001). This was observed for overall score, individual components, as well as when evaluated by LIFI tertiles of risk (Table 5). Similarly, LIFI was also the only score to significantly predict long-term mortality at 1- (HR 1.05 [1.04, 1.07] p < 0.001), 3- (HR 1.05 [1.04, 1.06] p < 0.001), and 5-years post-LT (1.04 [1.03, 1.06] p < 0.001); respectively (Table 5). Overall patient survival was also evaluated by Kaplan-Meier survival analysis of liver transplant patients stratified by low, moderate, and high-risk groups, based on previously reported stratifications (Figure 2). Risk stratification by LIFI, but not UCLA-FRS, P-SOFT, SOFT, BAR, or MELD 3.0, significantly correlated with patient survival following transplant. Finally clinical risk scores were assessed for discrimination power of 1-year mortality, using the C-statistic. LIFI showed superior discrimination (highest C-statistic) of post-LT mortality when compared to all other risk scores, irrespective of biologic MELD. This was especially pronounced in patients with MELD<30 (C-statistics=0.92) (Table 6).
Table 5.
Cox proportional hazard analysis of pre-transplant risk scores and post-transplant mortality
| MELD | 1.01 (0.96, 1.06), p=0.76 | 1.01 (0.97, 1.05), p=0.58 | 1.00 (0.98, 1.03), p=0.79 | 1.01 (0.99, 1.03), p=0.46 |
| MELD-Na | 1.01 (0.95, 1.06), p=0.79 | 1.01 (0.97, 1.05), p=0.58 | 1.00 (0.98, 1.03), p=0.76 | 1.01 (0.99, 1.03), p=0.44 |
| MELD 3.0 | 1.00 (0.95, 1.06), p=0.33 | 1.01 (0.97, 1.05), p=0.75 | 1.00 (0.98, 1.03), p=0.83 | 1.01 (0.98, 1.03), p=0.48 |
| D-MELD | 1.00 (1.00, 1.00), p=0.37 | 1.00 (1.00, 1.00), p=0.39 | 1.00 (1.00, 1.00), p=0.76 | 1.00 (1.00, 1.00), p=0.63 |
| MELD-GRAIL | 1.02 (0.88, 1.19), p=0.77 | 1.02 (0.92, 1.15), p=0.67 | 1.01 (0.94, 1.09), p=0.79 | 1.02 (0.95, 1.09), p=0.54 |
| MELD-GRAIL-Na | 1.02 (0.87, 1.20), p=0.76 | 1.03 (0.91, 1.15), p=0.66 | 1.01 (0.93, 1.09), p=0.78 | 1.02 (0.95, 1.10), p=0.53 |
| UCLA-FRS | 1.04 (0.95, 1.15), p=0.48 | 1.02 (0.95, 1.09), p=0.60 | 1.01 (0.96, 1.06), p=0.78 | 1.01 (0.97, 1.06), p=0.52 |
| BAR | 1.02 (0.92, 1.14), p=0.68 | 1.03 (0.95, 1.12), p=0.46 | 1.02 (0.96, 1.08), p=0.54 | 1.04 (0.99, 1.09), p=0.15 |
| SOFT | 1.01 (0.94, 1.07), p=0.87 | 1.03 (0.98, 1.07), p=0.23 | 1.02 (0.99, 1.05), p=0.23 | 1.02 (1.00, 1.05), p=0.08 |
| P-SOFT | 1.01 (0.94, 1.08), p=0.79 | 1.03 (0.98, 1.08), p=0.23 | 1.02 (0.99, 1.06), p=0.20 | 1.03 (1.00, 1.06), p=0.06 |
| LDRI | 0.69 (0.09, 5.04), p=0.72 | 1.09 (0.31, 3.81), p=0.89 | 1.10 (0.46, 2.66), p=0.83 | 1.05 (0.48, 2.29), p=0.90 |
| 1.07 (1.01, 1.13), p=0.02 | 1.06 (1.02, 1.11), p=0.003 | 1.05 (1.02, 1.07), p=0.001 | ||
| 1.05 (1.02, 1.07), p=0.001 | 1.05 (1.03, 1.07, p<0.001 | 1.05 (1.03, 1.06), p<0.001 | ||
| Fractalkine | 1.07 (1.03, 1.11), p<0.001 | 1.08 (1.05, 1.11), p<0.001 | 1.07 (1.04, 1.09), p<0.001 | 1.06 (1.04, 1.09), p<0.001 |
| LIFI Risk Group | ||||
| (reference) | (reference) | (reference) | ||
| 7.73 (1.42, 42.18) p=0.02 | 9.24 (2.39, 35.74) p=0.001 | 4.27 (1.98, 9.22) p<0.001 |
CI, confidence interval; MELD, Model for End-Stage Liver Disease; GRAIL, GFR assessment in liver disease; UCLA-FRS, University of California Los Angeles Futility Risk Score; BAR, balance of risk; SOFT, survival outcomes following liver transplantation; P-SOFT, predicted survival outcomes following liver transplantation; LDRI, liver donor risk index; LIFI, liver immune frailty index.
Figure 2. Overall patient survival following liver transplant for patients stratified by pre-transplant risk scores.
Patients included in this study were stratified by previously reported risk groups or tertiles for (A) LIFI, (B) UCLA-FRS, (C) P-SOFT, (D) SOFT, (E) BAR, and (F) MELD 3.0. Of these, only LIFI stratification resulted in significant correlation with post-transplant survival.
Table 6.
Discrimination power of clinical risk scores compared with LIFI in predicting 1-year post-LT mortality
| Risk scores | All patients (N=289) | MELD <30 (n=131) | MELD ≥30 (n=158) | |||
|---|---|---|---|---|---|---|
| C-statistic (95% CI) | p value | C-statistic (95% CI) | p value | C-statistic (95% CI) | p value | |
| LIFI | 0.85 (0.75, 0.96) | -- | 0.92 (0.85, 0.98) | -- | 0.81 (0.63, 0.98) | -- |
| CCI | 0.62 (0.50, 0.74) | 0.01 | 0.48 (0.32, 0.64) | <0.001 | 0.69 (0.54, 0.84) | 0.30 |
| SOFT | 0.58 (0.43, 0.73) | 0.01 | 0.50 (0.24, 0.75) | 0.002 | 0.63 (0.48, 0.78) | 0.12 |
| D-MELD | 0.56 (0.44, 0.67) | <0.001 | 0.65 (0.52, 0.78) | <0.001 | 0.52 (0.33, 0.70) | 0.01 |
| LDRI | 0.56 (0.45, 0.68) | <0.001 | 0.63 (0.52, 0.75) | <0.001 | 0.49 (0.33, 0.66) | 0.02 |
| BAR | 0.56 (0.42, 0.71) | 0.003 | 0.53 (0.31, 0.75) | <0.001 | 0.59 (0.41, 0.77) | 0.06 |
| MELD | 0.55 (0.40, 0.70) | 0.003 | 0.53 (0.38, 0.68) | <0.001 | 0.62 (0.42, 0.81) | 0.10 |
| MELD-Na | 0.55 (0.40, 0.70) | 0.003 | 0.52 (0.36, 0.68) | <0.001 | 0.61 (0.41, 0.81) | 0.09 |
| UCLA-FRS | 0.55 (0.40, 0.69) | 0.001 | 0.52 (0.34, 0.70) | <0.001 | 0.58 (0.42, 0.75) | 0.02 |
| MELD-GRAIL | 0.54 (0.39, 0.68) | 0.001 | 0.51 (0.35, 0.68) | <0.001 | 0.56 (0.37, 0.76) | 0.048 |
| Meld GRAIL-Na | 0.54 (0.39, 0.68) | 0.001 | 0.51 (0.34, 0.69) | <0.001 | 0.57 (0.37, 0.76) | 0.049 |
| MELD 3.0 | 0.53 (0.38, 0.67) | 0.001 | 0.54 (0.38, 0.70) | <0.001 | 0.56 (0.35, 0.76) | 0.06 |
CI, confidence interval; LIFI, Liver Immune Frailty Index; CCI, Charlson Comorbidity Index, age-adjusted; MELD, Model for End-Stage Liver Disease; UCLA-FRS, University of California Los Angeles Futility Risk Score; BAR, Balance of Risk score, SOFT, Survival Outcome Following Liver Transplantation; LDRI, Liver Donor Risk Index
Discussion
The “Final Rule” dictates allocation of livers in order of decreasing medical urgency (i.e. sickest first) while avoiding futile liver transplantation (LT) (2,3). The current allocation system in the US stratifies pre-transplant illness severity based on the MELD score, which predicts three month waitlist mortality with C-statistic of 0.78–0.87 (4,5). MELD, however, is a poor predictor of post-transplant mortality (C-statistic=0.44–0.53) (6,30). Other previously described pre-LT clinical scoring models either do not correlate with outcomes or require knowledge of donor and intraoperative information for calculation, which are not known prior to donor allocation. Multiple models have been described, all with c-statistic ≤ 0.7 for prediction of post-LT outcomes. Rising organ demand in conjunction with increasing recipient severity of illness necessitates a reliable method to risk-stratify critically ill patients based on their pre-LT severity of illness to avoid futile liver transplantation.
We have previously described the Liver Immune Frailty Index (LIFI), a biomarker panel based on HCV IgG status and plasma levels of MMP-3 and Fractalkine, which quantifies pre-LT immune dysfunction (a.k.a., immune frailty) and predicts risk of post-LT futility (23). Whether this model outperforms other conventional clinical scoring models was not known. Here, we find that LIFI significantly correlates with liver transplant recipient mortality at 6-months, as well as at 1-, 3-, and 5-years post-transplant. In addition, LIFI shows superior discrimination (highest C-statistic) of 1-year post-LT mortality compared to all other risk scores, regardless of biologic MELD.
MELD and other conventional clinical scoring tools rely on laboratory values as surrogates for illness severity (8–15,17,18); however, these disregard the immunological status of patients at the time of LT. Infection is the leading cause of mortality within the first year following liver transplant, and ongoing infection risk likely results from persistent immune dysfunction following liver transplant. Pretransplant immune dysfunction in cirrhosis arises from the physiologic and metabolic alterations associated with progressive liver decompensation. This leads to cirrhosis associated immune dysfunction (CAID), which is characterized by deficiency in both innate and adaptive immunity, resulting from chronic immune system stimulation of liver injury, pathogenic infections, and gut-derived antigens (31). Chronic immune stimulation and exhaustion of metabolic substrates ultimately induces an inappropriate compensatory anti-inflammatory response. In the setting of severe decompensation, cirrhotic patients exhibit impaired immune response to bacterial challenge, which can result in severe systemic infection, multi-organ failure, and short-term mortality (32,33). In its most severe form, immune frailty, pre-transplant immune dysfunction likely persists post-transplant and is exacerbated by immunosuppressive medications.
Prior clinical scoring systems have failed to capture the risk imparted by this severe state of ongoing immune dysfunction. This is a critical flaw that limits their clinical utility, as, infection is the leading cause of early post-transplant mortality. Of previously described models, three have shown the best sensitivity and specificity for predicting post-LT outcomes. These include the SOFT, BAR, and the UCLA-FRS scores (13,14,17). The SOFT score (14,18) and BAR score (6) were created from patient-level data from the UNOS database, which despite its statistical power, lacks granularity to capture variables of immune dysfunction and infection risk. In addition, both scores require knowledge of donor characteristics and fail to consider recipient comorbidities, which are critical risk factors considered before waitlist placement (34). For that purpose, the UCLA-FRS score was created. This index was created through retrospective assessment of single center data, albeit at the center with the largest longitudinal liver transplant experience in the US. The single-center study design improved granularity, allowing inclusion of comorbidity history through adjusted Charlson comorbidity index (CCI) and cardiac risk. In addition, it is the only score to include any markers of pretransplant immune dysfunction; as, pre-transplant sepsis within 30 days of transplant likely re ects immune dysregulation (17). The original derivation of the UCLA-FRS, however, included only patients with MELD ≥ 40. Follow-up validation studies have demonstrated subpar performance in patients with lower pre-transplant severity of illness (threshold of MELD at 30, c-statistic of 0.65) (6). Thus, an objective and replicable system which considers immune dysfunction is necessary to improve pre-transplant risk-stratification of post-LT mortality.
Our recently described LIFI score stratifies patients into high-, moderate-, and low-risk of post-LT mortality. Patients with high-LIFI had a 1-year post-LT mortality of 58.3% compared to 1.4% in low-LIFI recipients (23). With a c-statistic of 0.84 in our cohort, LIFI is emerging as a potentially superior tool to support and guide clinical decision-making to avoid futile outcomes in high-risk LT recipients. Of note, LIFI offers superior discrimination of patient risk of mortality regardless of pre-LT MELD. Other clinical models have failed to accurately forecast outcomes in the low MELD cohort. Patients receiving liver transplant at lower MELD scores commonly have MELD exceptions, allowing their waitlist prioritization, with exception most commonly being granted for hepatocellular carcinoma. This may suggest that LIFI is able to discriminate not only the risk of mortality due to immune dysfunction relating to sepsis, but LIFI may also correlate with the risk of mortality related to recurrent cancer. Given that immune dysregulation allows tumor cells to escape immune surveillance, persistent immune dysfunction following liver transplant may increase a recipient’s risk of developing de novo or recurrent disease. Additional studies are necessary to delineate this relationship further.
The relevance of pre-transplant metrics for prediction of post-liver transplant futility are especially relevant given the current public policy debate in the US as to whether predictors of post-liver transplant outcomes should be considered in candidate wait-list stratification. Machine learning algorithms, such as those employed in the optimized prediction of mortality (OPOM) model (35), may improve upon the less complex clinical scoring metrics presented here. Our data, however, clearly suggest that recipient biologic variables are strongly predictive of post-transplant outcomes, unlike scores comprising recipient clinical comorbidities, which seem barely better than a flip of a coin. This includes MELD 3.0, which will soon be implemented for liver transplant candidate waitlist prioritization. The inclusion of inaccurate clinical predictive metrics might risk unnecessary exclusion of patients from transplant that would otherwise greatly benefit from. Although LIFI could offer an objective predictive measure of immune function and post-transplant mortality, further multi-center validation and assessment of its serial progression over time in waitlisted patients is necessary. With further validation, LIFI may still not be appropriate for listing prioritization; as, there are too many variables at play for any single model to accurately predict outcomes in all patients. Instead, biologic criteria, such as the LIFI score, should be assessed at the center level and should not be included in national criteria for waitlist stratification.
There are several limitations to our findings. First, the LIFI was internally validated using granular patient-level data and immunologic assessment from patients at only two transplant centers. In addition, the LIFI was calculated via boot-strapping techniques, which does not consider changes in patient population while modeling prediction (36,37). A large multi-center validation cohort is necessary to verify the model. In addition, due to the use of a limited patient cohort, we were not able to perform a multivariate prediction of 1-year post-LT mortality using components of the different pre-transplant scoring models given the small numbers of events at 1 year. LIFI includes HCV IgG status in its calculation. HCV likely figures more heavily into the risk score given that the discovery cohort used spanned the era of introduction of direct-acting antiviral medication when transplant was more common for HCV. As patient demographics change, we may see an era effect in significantly associated immune biomarkers, resulting in LIFI score adjustment. Finally, there is potential for selection bias given that certain subgroups were excluded during creation of LIFI, including re-transplant recipients, patients of advance age, and patients with fulminant hepatic failure. Additional analysis is necessary to evaluate LIFI in these cohorts.
In conclusion, LIFI predicts patient survival and is the only score to significantly correlate with mortality in both high and low MELD recipients. Pre-LT assessment of immune dysregulation may be critical in predicting mortality after LT and may optimize selection of candidates with lowest risk of futile outcomes.
Table 3.
Post-transplant mortality and morbidity stratified by MELD score
| (N=289) | (n=131) | (n=158) | ||
|---|---|---|---|---|
| Post-transplant mortality | ||||
| Recipient COD | ||||
| Unknown/Other | 5 (9.8) | 2 (8.7) | 3 (10.7) | |
| Renal Failure | 1 (2.0) | 0 (0.0) | 1 (3.6) | |
| Sepsis/Infection | 19 (37.3) | 5 (21.7) | 14 (50.0) | |
| Cardiac | 4 (7.8) | 1 (4.3) | 3 (10.7) | |
| Graft Failure | 6 (11.8) | 2 (8.7) | 4 (14.3) | |
| Malignancy/Other | 2 (3.9) | 2 (8.7) | 0 (0.0) | |
| Recurrent HCC | 7 (13.7) | 6 (26.1) | 1 (3.6) | |
| Pulmonary | 1 (2.0) | 0 (0.0) | 1 (3.6) | |
| Neurologic | 3 (5.9) | 3 (13.0) | 0 (0.0) | |
| Suicide | 1 (2.0) | 1 (4.3) | 0 (0.0) | |
| GVHD | 1 (2.0) | 0 (0.0) | 1 (3.6) | |
| PTLD | 1 (2.0) | 1 (4.3) | 0 (0.0) | |
| Patient Death < 90d | 3 (1.0) | 2 (1.5) | 1 (0.6) | 0.46 |
| Patient Death < 1yr | 20 (6.9) | 9 (6.9) | 11 (7.0) | 0.98 |
| LOS (days), median (IQR) | 17.0 (11.0, 28.0) | 12.0 (9.0, 20.0) | 21.0 (15.0, 34.0) | <0.001 |
| Any Blood Transfusion | 123 (75.0) | 40 (63.5) | 83 (82.2) | |
| MI | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cardiomyopathy | 5 (3.0) | 1 (1.6) | 4 (3.9) | |
| Sepsis, 30 days | 36 (12.5) | 9 (6.9) | 27 (17.1) | |
| Pneumonia, 30 days | 42 (14.5) | 8 (6.1) | 34 (21.5) | |
| Abdominal infection, 30 days | 26 (9.0) | 5 (3.8) | 21 (13.3) | |
| UTI, 30 days | 42 (14.5) | 11 (8.4) | 31 (19.6) | |
| Positive culture, 30 days | 122 (42.2) | 34 (26.0) | 88 (55.7) | |
| Sepsis, 90 days | 48 (16.6) | 15 (11.5) | 33 (20.9) | |
| Pneumonia, 90 days | 57 (19.7) | 15 (11.5) | 42 (26.6) | |
| Abdominal infection, 90 days | 38 (13.1) | 10 (7.6) | 28 (17.7) | |
| UTI, 90 days | 69 (23.9) | 23 (17.6) | 46 (29.1) | |
| Severe Infection, 90 days | 128 (44.3) | 44 (33.6) | 84 (53.2) | |
| Positive culture, 90 days | 158 (54.7) | 54 (41.2) | 104 (65.8) | <0.001 |
IQR, interquartile range; COD, cause of death; HCC, hepatocellular carcinoma; GVHD, graft versus host disease; PTLD, post-transplant lymphoproliferative disease; LOS, length of hospitalization; MI, myocardial infarction; UTI, urinary tract infection; PostTx, post-transplant.
Acknowledgements
The authors would like to thank Julie Corkrean, Julius Balough, and Jane Thomas for assistance with patient data collection. A pre-print version of this project is available online (38).
Funding
This work was supported, in part, through grants from the NIH NIDDK (K08DK118187), the Association of Women Surgeons, the New Jersey Health Foundation, and the American Society of Transplant Surgeons-Transplant Genomics Institute.
Footnotes
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board (IRB) at both, Houston Methodist Hospital (HMH) and University Hospital/Rutgers New Jersey Medical School (Rutgers NJMS). The participants provided their written informed consent to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Files
Contributor Information
J. Esli Medina-Morales, Rutgers New Jersey Medical School.
Guergana G. Panayotova, Rutgers New Jersey Medical School
Duc T. Nguyen, Houston Methodist Research Institute
Edward A. Graviss, Houston Methodist Research Institute
Gagan S. Prakash, Rutgers New Jersey Medical School
Jeffery A. Marsh, Rutgers New Jersey Medical School
Sopio Simonishvili, Rutgers New Jersey Medical School.
Yash Shah, Rutgers New Jersey Medical School.
Tumininu Ayorinde, Rutgers New Jersey Medical School.
Yong Qin, Rutgers New Jersey Medical School.
Lianhua Jin, Rutgers New Jersey Medical School.
Theofano Zoumpou, Rutgers New Jersey Medical School.
Laurie J. Minze, Rutgers New Jersey Medical School
Flavio Paterno, Rutgers New Jersey Medical School.
Arpit Amin, Rutgers New Jersey Medical School.
Grace Lee Riddle, Rutgers New Jersey Medical School.
R. Mark Ghobrial, Houston Methodist Hospital.
James V. Guarrera, Rutgers New Jersey Medical School
Keri E. Lunsford, Rutgers New Jersey Medical School
Data availability statement
The deidentified raw data supporting the conclusions of this article will be made available by the authors, without undue reservation upon request.
References
- 1.Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020. Jan 1;20:193–299. [DOI] [PubMed] [Google Scholar]
- 2.Code of Federal Regulation (CFR) annual edition, Part 121 - Organ Procurement and Transplantationation Network. Sect. Chapter I, Subchapter K.
- 3.Neuberger J. What is the real gain after liver transplantation? Liver Transpl. 2009. Nov;15 Suppl 2:S1–5. [DOI] [PubMed] [Google Scholar]
- 4.Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002. Sep;8(9):851–8. [DOI] [PubMed] [Google Scholar]
- 5.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001. Feb;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel A, Linecker M, Kron P, Györi G, De Oliveira ML, Müllhaupt B, et al. Risk Assessment in High-and Low-MELD Liver Transplantation. Am J Transplant. 2017. Apr;17(4):1050–63. [DOI] [PubMed] [Google Scholar]
- 7.Kim WR, Lindor KD, Malinchoc M, Petz JL, Jorgensen R, Dickson ER. Reliability and validity of the NIDDK-QA instrument in the assessment of quality of life in ambulatory patients with cholestatic liver disease. Hepatology. 2000. Nov;32(5):924–9. [DOI] [PubMed] [Google Scholar]
- 8.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008. Sep 4;359(10):1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, et al. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021. Dec;161(6):1887–1895.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asrani SK, Jennings LW, Trotter JF, Levitsky J, Nadim MK, Kim WR, et al. A Model for Glomerular Filtration Rate Assessment in Liver Disease (GRAIL) in the Presence of Renal Dysfunction. Hepatology. 2019. Mar;69(3):1219–30. [DOI] [PubMed] [Google Scholar]
- 11.Asrani SK, Jennings LW, Kim WR, Kamath PS, Levitsky J, Nadim MK, et al. MELD-GRAIL-Na: Glomerular Filtration Rate and Mortality on Liver-Transplant Waiting List. Hepatology. 2020. May;71(5):1766–74. [DOI] [PubMed] [Google Scholar]
- 12.Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009. Feb;9(2):318–26. [DOI] [PubMed] [Google Scholar]
- 13.Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, et al. Are There Better Guidelines for Allocation in Liver Transplantation?: A Novel Score Targeting Justice and Utility in the Model for End-Stage Liver Disease Era. Ann Surg. 2011. Nov;254(5):745. [DOI] [PubMed] [Google Scholar]
- 14.Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, et al. Survival Outcomes Following Liver Transplantation (SOFT) Score: A Novel Method to Predict Patient Survival Following Liver Transplantation [Internet]. Vol. 8, American Journal of Transplantation. 2008. p. 2537–46. Available from: 10.1111/j.1600-6143.2008.02400.x [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006. Apr;6(4):783–90. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 17.Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014. Jun;259(6):1186–94. [DOI] [PubMed] [Google Scholar]
- 18.Rana A, Jie T, Porubsky M, Habib S, Rilo H, Kaplan B, et al. The survival outcomes following liver transplantation (SOFT) score: validation with contemporaneous data and stratification of high-risk cohorts. Clin Transplant. 2013. Jul 1;27(4):627–32. [DOI] [PubMed] [Google Scholar]
- 19.Ravaioli M, Grande G, Di Gioia P, Cucchetti A, Cescon M, Ercolani G, et al. Risk Avoidance and Liver Transplantation: A Single-center Experience in a National Network. Ann Surg. 2016. Nov;264(5):778–86. [DOI] [PubMed] [Google Scholar]
- 20.Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011. Jul 12;58(3):223–31. [DOI] [PubMed] [Google Scholar]
- 21.Xia VW, Taniguchi M, Steadman RH. The changing face of patients presenting for liver transplantation. Curr Opin Organ Transplant. 2008. Jun;13(3):280–4. [DOI] [PubMed] [Google Scholar]
- 22.Rana A, Ackah RL, Webb GJ, Halazun KJ, Vierling JM, Liu H, et al. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades [Internet]. Vol. 269, Annals of Surgery. 2019. p. 20–7. Available from: 10.1097/sla.0000000000002650 [DOI] [PubMed] [Google Scholar]
- 23.Panayotova GG, Simonishvili S, Nguyen DT, Graviss EA, Aware N, Manner CJ, et al. Identification and internal validation of a novel pre-transplant biomarker panel to predict mortality following liver transplantation: The Liver Immune Frailty Index [Internet]. Research Square. 2022. Available from: https://www.researchsquare.com/article/rs-2134172/latest.pdf [Google Scholar]
- 24.Ayorinde T, Panayotova G, Sharma A, Lunsford KE. Clinical and biomarker assessment of frailty in liver transplantation. Curr Opin Organ Transplant. 2021. Oct 1;26(5):488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mysore KR, Ghobrial RM, Kannanganat S, Minze LJ, Graviss EA, Nguyen DT, et al. Longitudinal assessment of T cell inhibitory receptors in liver transplant recipients and their association with posttransplant infections. Am J Transplant. 2018. Feb;18(2):351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaltenborn A, Hartmann C, Salinas R, Ramackers W, Kleine M, Vondran FWR, et al. Risk factors for short- and long-term mortality in liver transplant recipients with MELD score ≥30. Ann Transplant. 2015. Jan 29;20:59–69. [DOI] [PubMed] [Google Scholar]
- 27.Schrem H. Value of the preoperative SOFT-score, P-SOFT-score, SALT-score and labMELD-score for the prediction of short-term patient and graft survival of high-risk liver transplant recipients with a pre-transplant labMELD-score ≥30 [Internet]. Vol. 17, Annals of Transplantation. 2012. p. 11–7. Available from: 10.12659/aot.883218 [DOI] [PubMed] [Google Scholar]
- 28.Human D. Declaration of Helsinki. Lancet. 2001. Jan 20;357(9251):236. [DOI] [PubMed] [Google Scholar]
- 29.Policy and system changes effective January 11, 2016, adding serum sodium to MELD calculation. https://unos.org/news/policy-and-system-changes-effective-january-11-2016-adding-serum-sodium-to-meld-calculation/.
- 30.Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017. Aug;66(2):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011. Sep;9(9):727–38. [DOI] [PubMed] [Google Scholar]
- 32.Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011. Sep;55(3):574–81. [DOI] [PubMed] [Google Scholar]
- 33.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986. Mar–Apr;6(2):252–62. [DOI] [PubMed] [Google Scholar]
- 34.Artru F, Samuel D. Approaches for patients with very high MELD scores. JHEP Reports. 2019;1(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertsimas D, Kung J, Trichakis N, Wang Y, Hirose R, Vagefi PA. Development and validation of an optimized prediction of mortality for candidates awaiting liver transplantation. Am J Transplant. 2019. Apr;19(4):1109–18. [DOI] [PubMed] [Google Scholar]
- 36.Bleeker SE, Moll HA, Steyerberg EW, Donders ART, Derksen-Lubsen G, Grobbee DE, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol. 2003. Sep;56(9):826–32. [DOI] [PubMed] [Google Scholar]
- 37.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999. Mar 16;130(6):515–24. [DOI] [PubMed] [Google Scholar]
- 38.Esli Medina-Morales J, Panayotova GG, Nguyen DT, Graviss EA, Prakash GS, Marsh JA, et al. Pre-transplant Biomarkers of Immune Dysfunction Improve Risk Assessment of Post-transplant Mortality Compared With Conventional Clinical Risk Scores. 2023. Feb 6 [cited 2023 Feb 10]; Available from: https://www.researchsquare.com/article/rs-2548184/v1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified raw data supporting the conclusions of this article will be made available by the authors, without undue reservation upon request.