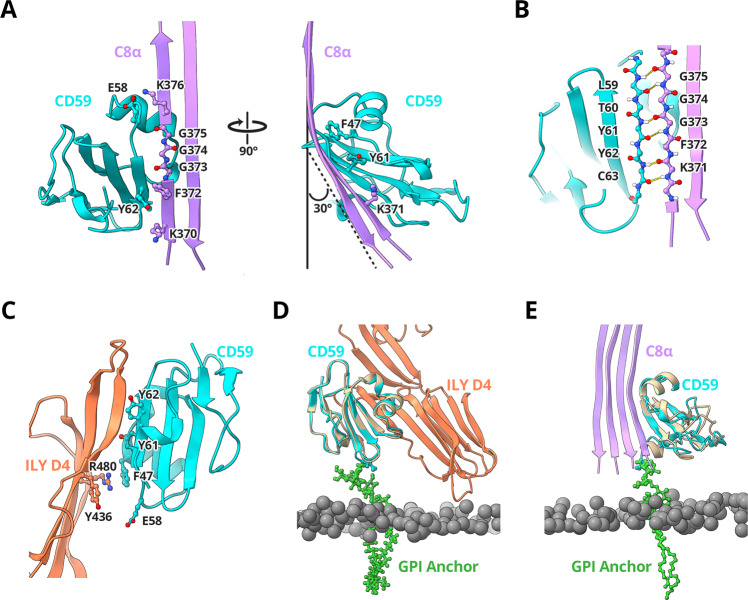
Fig. 3. CD59 interaction interfaces.
A Ribbon diagram of the C8α-CD59 interface. CD59 (cyan) captures the extending TMH2 residues of C8α (pink). Aromatic residue CD59:F47 binds C8α glycines (G373, G374, G375) to bend the β-hairpin trajectory. The C8α-CD59 interface is further stabilized by a salt bridge between CD59:E58 and C8α:K376, together with a pair of consecutive tyrosine-lysine interactions on either side of the β-sheet (CD59:Y62-C8α:K370; CD59:Y61-C8α:K371). Key residues that mediate the interactions are shown as sticks. B Hydrogen-bonding pattern of backbone atoms within the intermolecular antiparallel β-sheet. C Ribbon diagram of the ILY-CD59 interaction interface (PDB ID: 5IMT)33. ILY (orange) binds CD59 (cyan) through a β-hairpin extension of domain 4 (D4). The ILY-CD59 interface is comprised of an intermolecular anti-parallel β-sheet, including CD59 residues that engage C8α. D Superposition of the ILY-CD59 crystal structure (PDB ID: 5IMT) with a pose from the atomistic molecular dynamics simulation of GPI-anchored CD59 (tan). E Superposition of the C5b8-CD59 structure with a different pose from the atomistic molecular dynamics simulation of GPI-anchored CD59 (tan). In this position, CD59 is rotated 106° relative to its position in (D). Phosphorous atoms from lipid headgroups are grey spheres, simulated GPI anchor for CD59 is shown in green sticks. Initial and final configurations for the three MD repeats are included in the Supplementary Data Files.