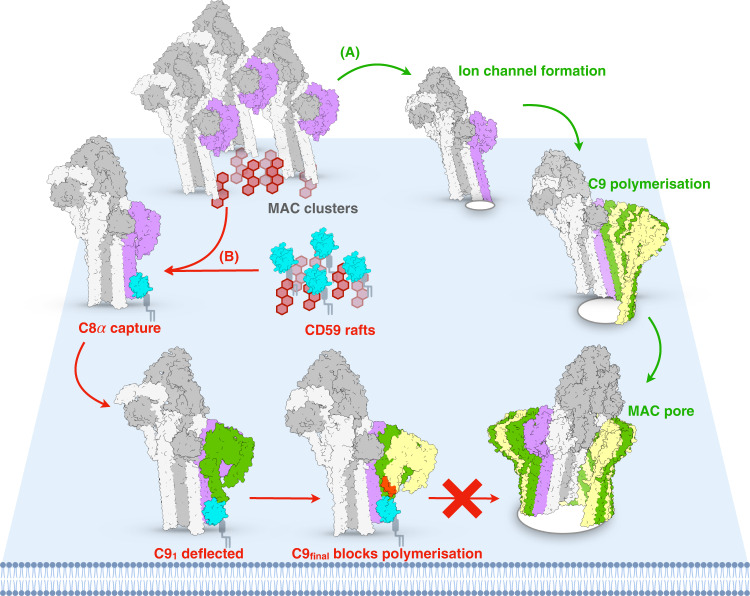
Fig. 6. Schematic of MAC assembly and its inhibition by CD59.
A MAC assembles on cholesterol-rich microdomains (red hexagons) in the plasma membrane. While C7 anchors the growing MAC, C8β thins the bilayer and primes the membrane for rupture by C8α (pink). C9 joins the assembly, undergoing discrete structural transitions to form the pore. The MACPF domain of soluble C9 makes an initial contact and aligns the central β-sheet. The pore-forming β-hairpins extend sequentially. TMH1 is followed by TMH2, which allow C9 polymerization to complete the MAC pore. B CD59 is a GPI-anchored cell surface receptor that clusters in cholesterol-rich microdomains. Upon complement activation, CD59 could respond to membrane thinning by C8β and capture C8α as its transmembrane β-hairpins are extending (pink). While bound to C8α, CD59 is positioned to deflect the cascading C9 β-hairpins and divert their membrane trajectory (green). The next C9 molecule (yellow) is able to bind but trapped in an intermediate conformation in which TMH2 remains helical (red) and in which C9 polymerization is halted.