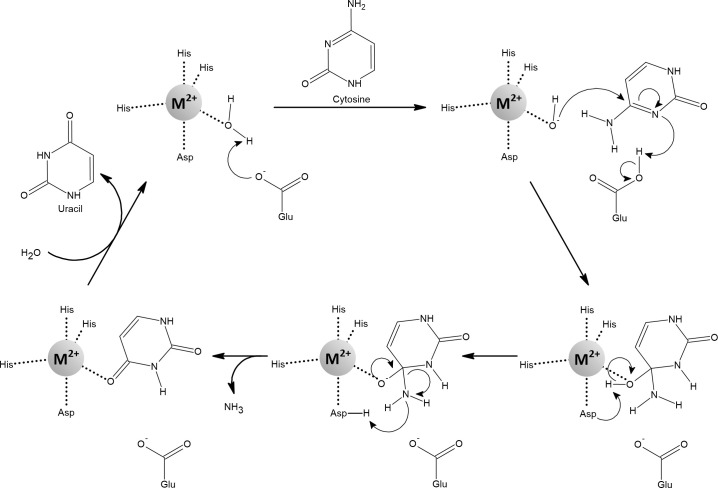
Figure 13.
The conversion of cytosine to uracil catalyzed by bacterial cytosine deaminase. The metal ion accommodates a trigonal-bipyramidal geometry with an exchangeable ligand that is a water molecule. A proton is removed from this coordinated water molecule and in the next step the metal-coordinated hydroxide nucleophilically attacks the substrate, simultaneously transferring a proton to the substrate. In the next step, the second proton is transferred from the original water molecule (now hydroxyl group) to the amine group, producing ammonia that leaves the substrate. This step is probably facilitated by the metal-coordinating aspartate. The new water molecule displaces the uracil. Various metals can facilitate this reaction, but iron is most effective (Porter and Austin, 1993; Ireton et al., 2002b; Hall et al., 2011; Anjem and Imlay, 2012; Manta et al., 2014).