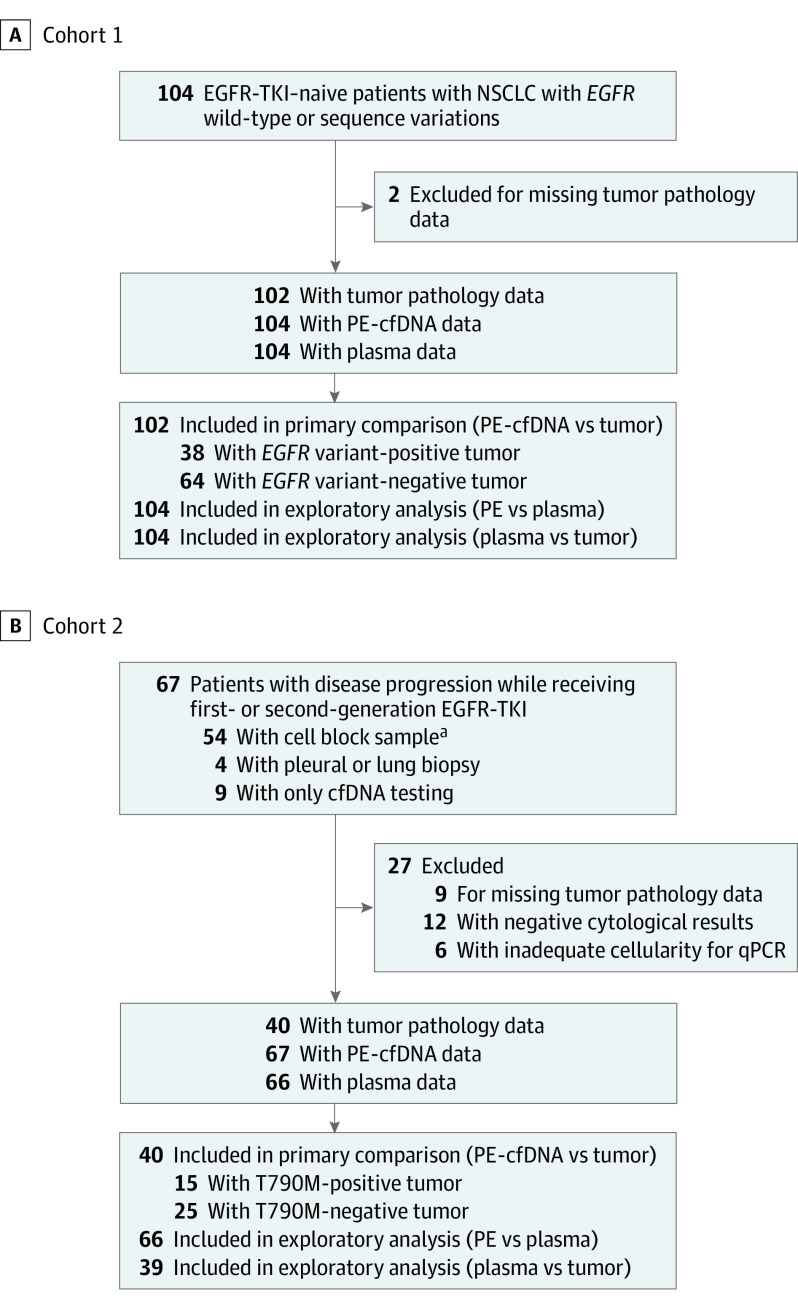
Figure 1. Patient Flowchart.
A, The preplanned sample size was 100, and 102 patients had matched PE-cfDNA and tissue for comparison. B, A separate matching analysis of the Thr790Met variant (T790M)–detection rate among 53 patients who had either negative (n = 12) or positive (n = 6) results on cytological testing of effusion but inadequate tumor DNA for molecular testing (n = 6) was performed to capture the T790M-detection rate by thoracocentesis or pericardiocentesis, which resembled actual clinical practice. cfDNA indicates cell-free DNA; EGFR-TKI, epidermal growth factor receptor–tyrosine kinase inhibitor; NSCLC, non–small cell lung cancer; PE-cfDNA, pleural and pericardial effusion cell-free DNA; and qPCR, quantitative polymerase chain reaction.
aOne patient had plasma EGFR data missing; thus, 53 were included in an exploratory analysis of T790M-detection rate in the cell block, plasma cfDNA, and PE-cfDNA samples.