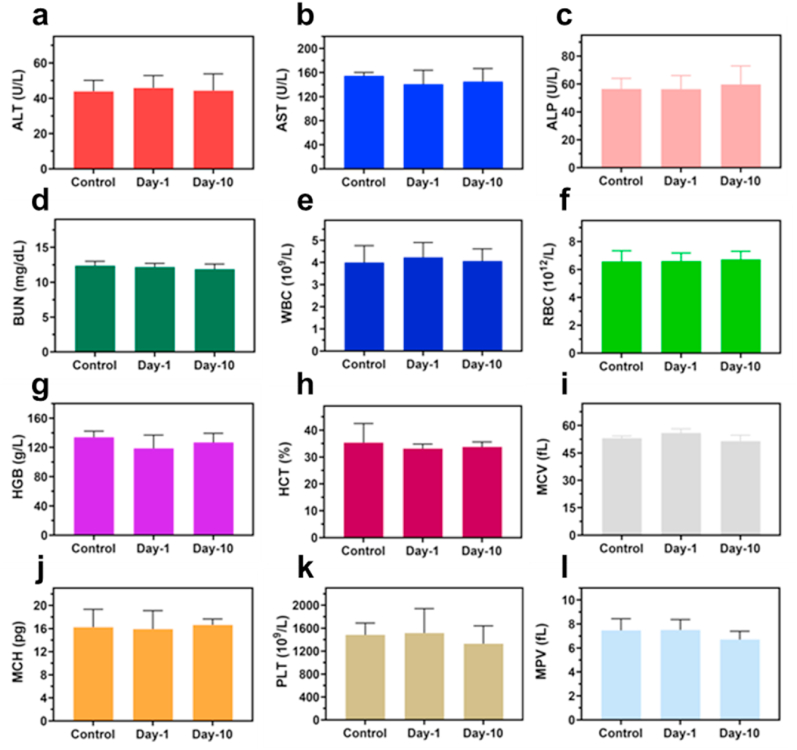
Fig. 8.
Biosafety evaluations of CAZ NPs in vivo. Blood biochemistry examination, including (a) ALT, (b) AST, (c) ALP, and (d) BUN. Blood routine examination, including (e) WBC, (f) RBC, (g) HGB, (h) HCT, (i) MCV, (j) MCH, (k) PLT, and (l) MPV of healthy mice after subcutaneous injection under different treatment conditions at day-0, day-1, and day-10.