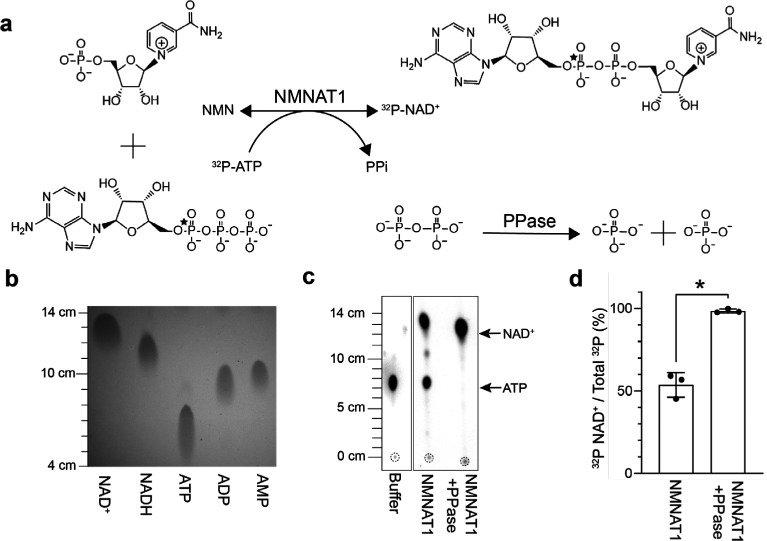
Figure 1.
PPase improved the yield of the NMN adenylyl transferase reaction in vitro. (a) Proposed reaction for the enzymatic synthesis of 32P-NAD+ from 32P-ATP. Position of the 32P label is indicated by a star. (b) NAD+, NADH, ATP, ADP, and AMP standards (>95% purity, 5 μL of 50 μM stocks) were resolved with thin layer chromatography (TLC) using PEI cellulose F plates and imaged following excitation at 254 nm. (c) Representative images of 1 μL from the enzymatic reactions being resolved with TLC and quantitated following exposure with a phosphorimaging screen. Leading edges were measured from the origins (dashed circles). (d) Calculated yields of 32P-NAD+ (mean ± SD, n = 3, Student’s t test, *p < 0.01).