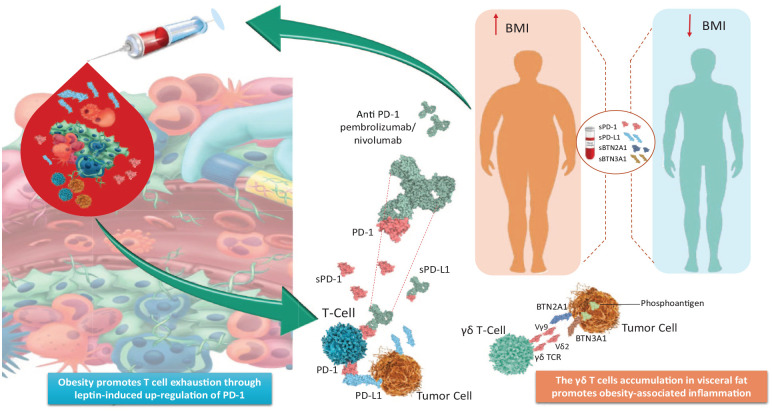
Figure 1.
A putative role of PD-1/PD-L1 axis and BTNs is highlighted as mechanism of cancer immune evasion and obesity-associated inflammation.
Obesity promotes T-cell exhaustion through leptin-induced upregulation of PD-1. This PD-1-mediated immune dysfunction in obesity cancer patients would be reversible by PD-1/PD-L1 inhibitors, making the tumors markedly more responsive to ICIs. The γδ T-cell accumulation in visceral fat promotes chronic inflammation. The Vγ9Vδ2+, the major class of γδ T cells, are activated by phosphoantigens produced by tumor cells, and needed the combination of two immunoglobulin superfamily members, BTN2A1 and BTN3A1. The soluble forms of PD-1, PD-L1, BTN2A1, and BTN3A1 can be detected in the peripheral blood. Circulating sICs/BTNs detection at baseline, before starting anti-PD-1 treatment, along with BMI, could give more insights into the symbiotic immune-metabolic interplay, to predict immunotherapy response.
BTNs, butyrophilins; ICIs, immune checkpoint inhibitors; sICs, soluble immune checkpoints; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.