Figure 1.
Infection-related modifications of pectins
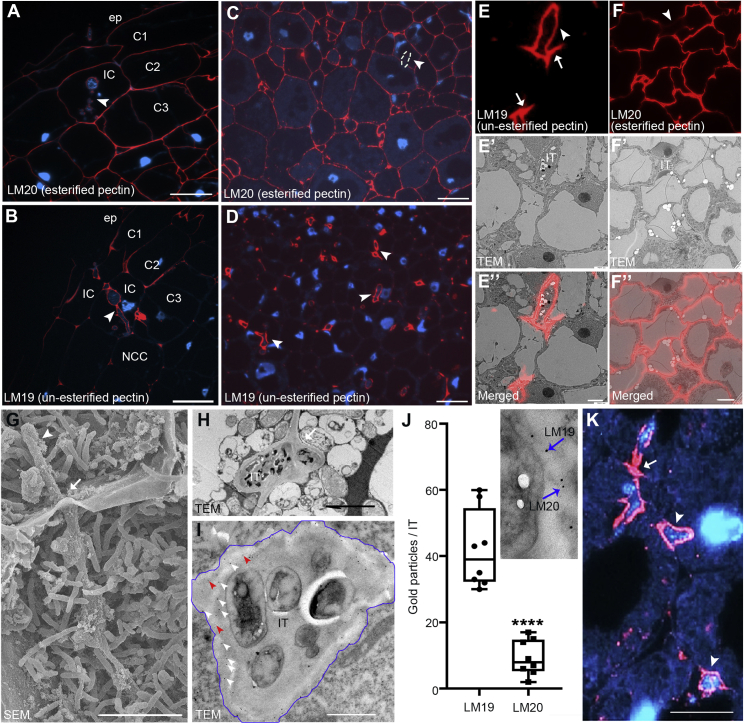
(A–D) Esterified pectins (LM20, red) are present on the walls of root cortical cells (A) and all cell types in 14-day-old Medicago nodules (C). They only weakly accumulate on the infection thread (IT; arrowheads and indicated by the white dashed line). Enrichment of un-esterified pectins (LM19, red) in infection threads in root cortical cells (B; arrowhead) and peripheral nodule cells with strong accumulations at nodular infection threads (D; arrowheads). Images in (A) and (B) were taken from consecutive sections from the same nodule but probed with two different antibodies. DNA was counterstained with DAPI (blue); ep, epidermis; C1, 1st cortical cell layer; C2, 2nd cortical cell layer; C3, 3rd cortical cell layer; IC, infected cell; NCC, non-colonized cell.
(E–F″) CLEM analysis of esterified (E)–(E″) and un-esterified (F)–(F″) pectins showing the fluorescence image after immuno-labeling of ultrathin sections (E) and (F), the corresponding TEM image (E′) and (F′) and the corresponding merged images (E″) and (F″). Nodular infection threads (IT) are indicated by arrowheads (E) and (F) and extensions of un-esterified pectin toward the direct neighboring cell at potential transcellular passage sites are labeled by arrows (E).
(G and H) Transcellular passage site (arrows) with a nodular infection thread (IT; arrowhead) passing from cell to cell as shown by scanning electron microscopy image (SEM) (G) and transmission electron microscopy (TEM) (H).
(I) Double immuno-gold labeling of un-esterified (LM19; 12 nm) and esterified (LM20; 5 nm) pectins at a nodular infection thread passage site showed labeling of the IT periphery. The IT periphery is encircled by a blue circle line; white and red arrowheads indicate immuno-gold labeled by LM19 and LM20, respectively.
(J) Quantification data for double immuno-gold labeling using LM19/LM20. Gold particles with different grain sizes were counted per IT (n = 8). Data are mean ± SE. Statistics were performed using an unpaired two-tailed t test: ∗∗∗∗p < 0.0001.
(K) Strong enrichment of Ca2+-complexed pectins (labeled by the 2F4 antibody) around ITs (arrowheads) and transcellular passage sites (arrow). DNA was counterstained with DAPI (blue). TEM, transmission electron microscopy; SEM, scanning electron microscopy image.
Scale bars, 50 μm in (A)–(D); 2,5 μm in (E)–(F″); 10 μm in (G), (H), (I), and (K).
See also Figure S1.