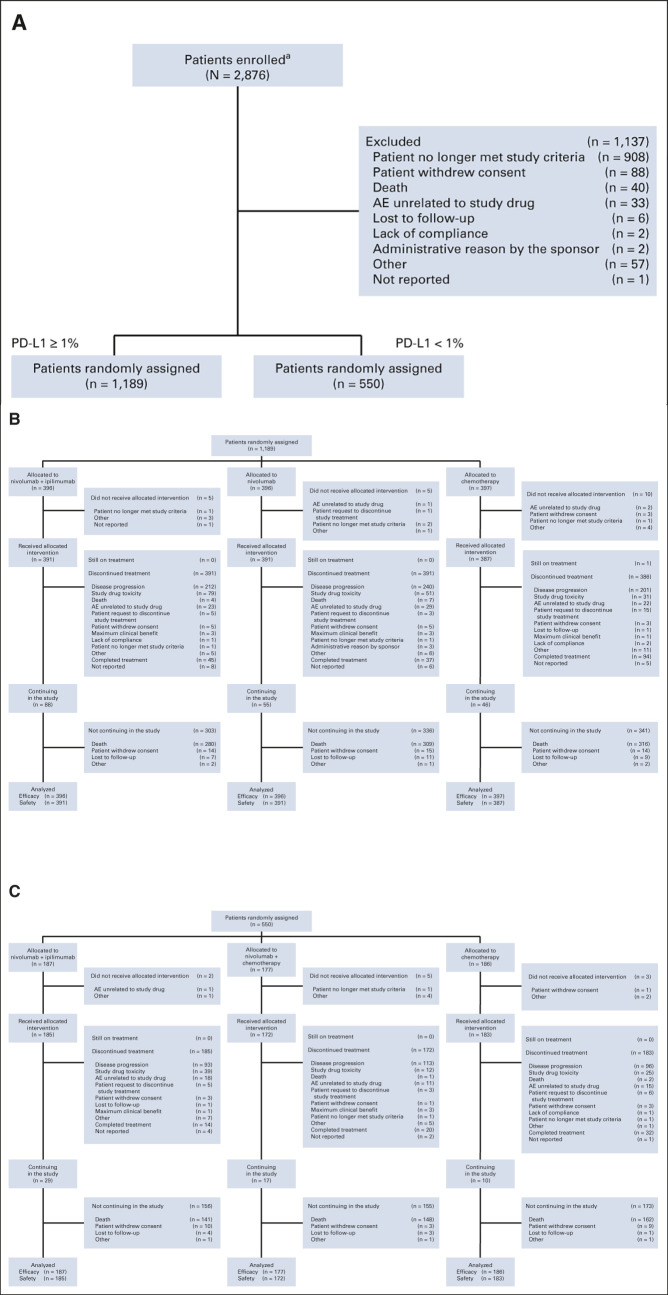
FIG 1.
CheckMate 227 CONSORT diagram. (A) Enrollment and allocation of patients with tumor PD-L1 expression ≥ 1% and < 1%, (B) disposition of patients with tumor PD-L1 expression ≥ 1%, and (C) disposition of patients with tumor PD-L1 expression < 1%. aOne patient was enrolled twice in error but not randomly assigned, not having met study criteria; the number of patients enrolled has been corrected here since the original report.10 AE, adverse event; CONSORT, Consolidated Standards of Reporting Trials; PD-L1, programmed death ligand 1. From the New England Journal of Medicine, Hellmann et al, Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer, 381:2020-2031, 2019 © Massachusetts Medical Society. Reprinted with permission from the Massachusetts Medical Society.10