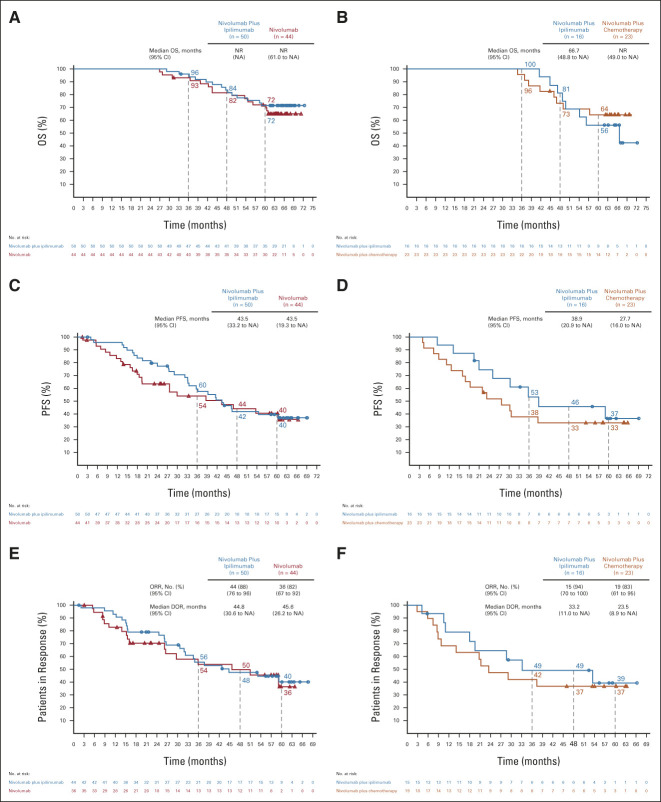
FIG 5.
OS, PFS, and ORR/DOR in patients who completed 2 years of immunotherapy by the tumor PD-L1 expression level. OS in patients with (A) tumor PD-L1 expression ≥ 1% or (B) tumor PD-L1 expression < 1%; PFS in patients with (C) tumor PD-L1 expression ≥ 1% or (D) tumor PD-L1 expression < 1%; ORR and DOR in patients with (E) tumor PD-L1 expression ≥ 1% or (F) tumor PD-L1 expression < 1%. Ninety-five percent CIs for the nivolumab plus ipilimumab and nivolumab (PD-L1 ≥ 1%) or nivolumab plus chemotherapy (PD-L1 < 1%) arms at 5-year landmarks, respectively: (A) 57 to 82 and 56 to 83, (B) 30 to 76 and 41 to 80, (C) 25 to 53 and 24 to 56, (D) 13 to 61 and 15 to 52, (E) 24 to 56 and 16 to 57, and (F) 14 to 64 and 17 to 57. DOR, duration of response; HR, hazard ratio; NA, not available; NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.