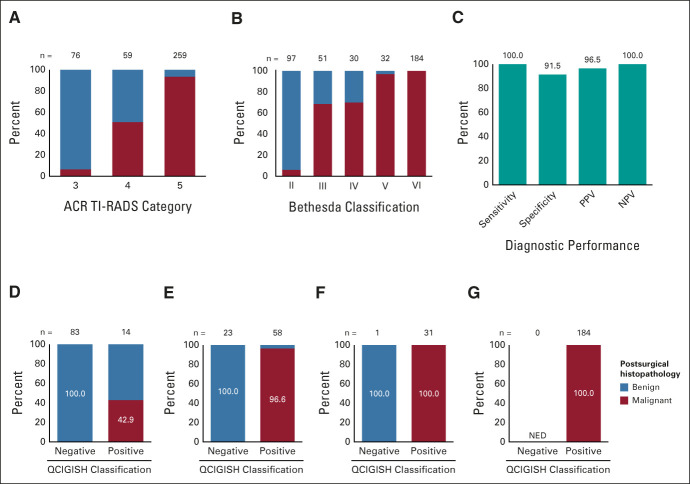
FIG 3.
Performance of QCIGISH test in the blinded prospective validation cohort. (A) Distribution of histopathologically benign and malignant cases in different ultrasound imaging categories. (B) Distribution of histopathologically benign and malignant cases in different Bethesda cytopathology categories. (C) Sensitivity, specificity, PPV, and NPV of the QCIGISH test in the blinded prospective validation study. Distribution of histopathologically benign and malignant cases in QCIGISH-negative and QCIGISH-positive groups for (D) Bethesda II, (E) Bethesda III and IV, (F) Bethesda V, and (G) Bethesda IV categories. Histopathologic benign and malignant diagnoses were confirmed by postsurgical thyroid examination in all cases. ACR TI-RADS, American College of Radiology Thyroid Imaging, Reporting, and Data System; NED, no evidence of disease; NPV, negative predictive value; PPV, positive predictive value; QCIGISH, quantitative chromogenic imprinted gene in situ hybridization.