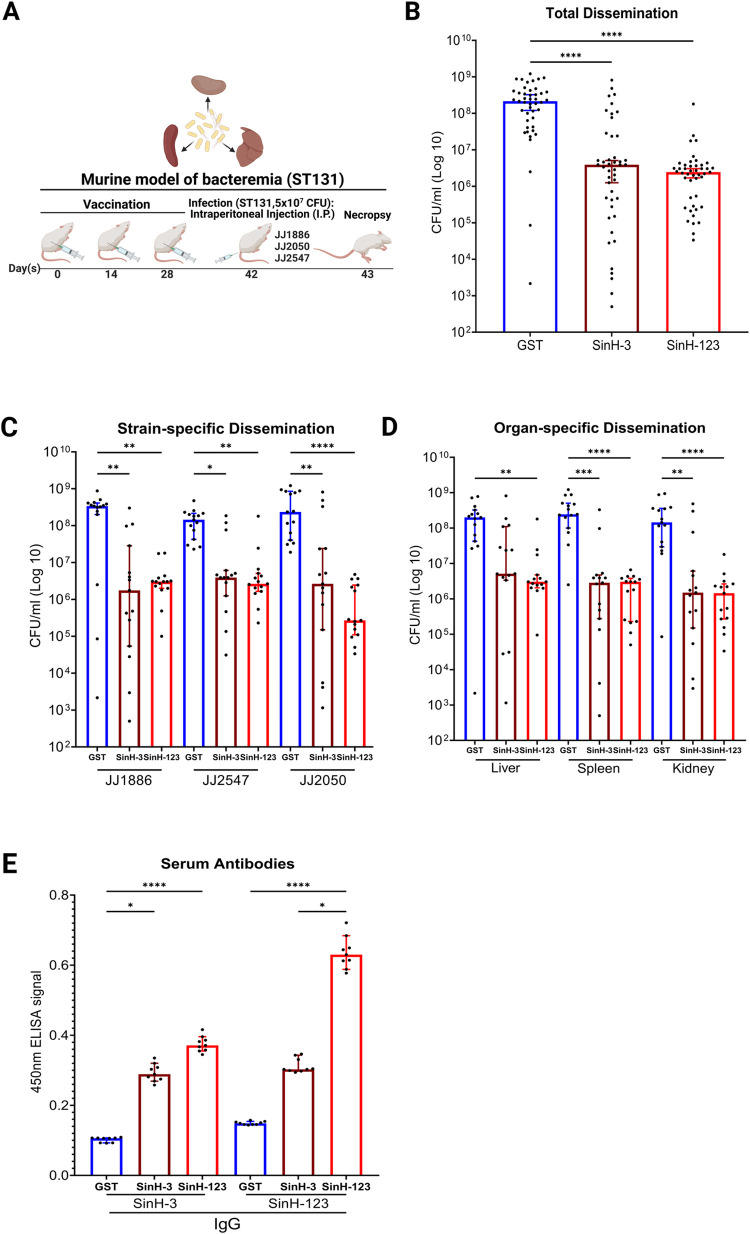
Fig 3. Assessment of the protective efficacy and immunogenicity of SinH-based vaccines against ExPEC sequence type 131 (ST131) bacteremia.
(A) The vaccination scheme was used in this experiment. BALB/cJ, 6 weeks old, female mice were subcutaneously immunized with SinH-based antigens (SinH-3, SinH-123, N = 15) or GST alone (N = 15) and injected with an intraperitoneal (IP) injection of 5 × 107 CFU of different ExPEC ST131 strains (JJ1886, JJ2547, JJ2050). Organs were harvested and plated to determine bacteria levels. Serum was taken from individual mice after immunization and ExPEC infection. The schematic diagram was made in BioRender. (B) Box-and-whisker plots of the bacterial levels (CFU/ml) in combining the counts from all organs (liver, spleen, kidney) and all ExPEC strains (JJ1886, JJ2547, JJ2050); (C) or the bacterial levels (CFU/ml) of each ExPEC ST131 strain in combining the counts from all organs; (D) or the bacterial levels (CFU/ml) of all ExPEC strains in each type of organ following necropsy. (E) ELISA analysis of sera from SinH-based antigens vaccinated animals using antigens, SinH-3 or SinH-123 (GST-tag removed), as the capture antigen. Error bars indicate the median with 95% confidence interval (CI). Significant was determined by theKruskal-Wallis analysis of variance (ANOVA) with Dunn’s multiple comparisons correction. Symbols represent data of individual mice. One star (*) P < 0.05, two stars (**) P < 0.01, three stars (***) P < 0.001, four stars (****) P < 0.0001. The Box-and-whisker plots were exported from Graphpad Prism 9 and annotated using BioRender.