|
Lipid-based nanocarriers
|
Liposomes
|
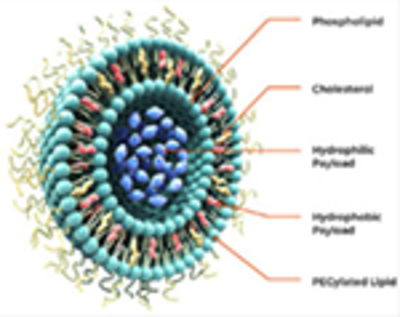
Size ranges from 50 nm to several micrometers. Spherical vesicles are composed of a lipid (amphipathic phospholipids) bilayer membrane arranged around an aqueous core (close resemblance to the mammalian cell). Drugs can be either entrapped inside the aqueous core (hydrophilic) or in the bilayer membrane (hydrophobic). Surface can be modified with modifiers, such as ligands or antibodies to form targeted liposomes. Lipids with special stimuli sensitivity (pH, temperature, light, etc.) can be formed stimuli-responsive liposomes.
|
The bilayer membrane components control pharmacokinetic properties such as elimination half-life, biodistribution, permeability, and drug release rate (Ait-Oudhia et al., 2014). Liposome encapsulation may reduce drug clearance by the immune and renal systems and prolong circulation time increasing drug availability (Bulbake et al., 2017). PEGylated liposomes enhance the circulation half-lives following systemic administration. Reduce drug degradation, limit potential off-target toxicity, and increase the concentration inside tumor cells, thus enhancing treatment efficacy. Conjugation with specific ligands increases efficiency and specifically target site-specific delivery. Delivery of drug combination to achieve an additive or synergistic efficacy.
|
Leakage and fusion of encapsulated drug/molecules. High production cost (Daraee et al., 2016). Rapid clearance of conventional liposomes (short circulating half-lives).
|
Doxil® (doxorubicin) Myocet—non-PEGylated liposomal for DOX VYXEOS® (Liposomal formulation of cytarabine: daunorubicin 5:1 M ratio) Talidox—novel liposomal DOX formulation ThermoDox®—heat-activated liposomal formulation of DOX (Nardecchia et al., 2019). EndoTAG-1—cationic liposomes of PTX LEP-ETU—liposomal formulation of PTX
|
Lipid nanoparticles (LNPs)
|
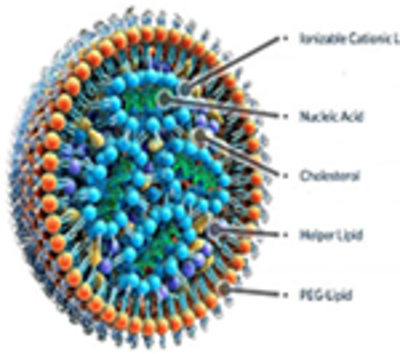
Next-generation liposomes with more complex internal lipid architecture and minimal internal aqueous presence than traditional liposomes. Cationic lipid NPs can encapsulate nucleic acid in synthetic positively charged lipids. Cubosomes are highly stable NPs formed from a lipid in the cubic phase and stabilized by a polymer-based outer corona.
|
Improves stability of nucleic acid by preventing degradation by nucleases and enabling passive diffusion across membranes Organ-specific delivery is based on the overall charge. Positively, neutrally, and negatively charged LNPs lead to deliver in the lung, liver, and spleen, respectively.
|
|
|
Solid lipid nanoparticles (SLNs)
|
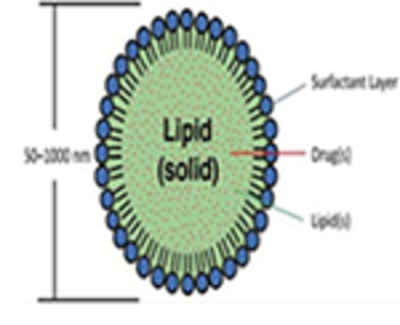
Size ranges from 50 to 1000 nm. Drug is molecularly dispersed within the lipid matrix, and the system is stabilized with an emulsifier. Lipids are solid at room temperatures (high melting triglycerides, complex glyceride mixtures, monoglycerides, hard fats, waxes, etc.). SLNs can carry a variety of therapeutics including small drug molecules (hydrophilic and lipophilic), large biomacromolecules (polysaccharides, etc.), genetic material (DNA/siRNA), and vaccine antigens too.
|
Improves the solubility of sparingly water-soluble drugs especially BCS Classes II and IV drugs. Protect active compounds from biological degradation or transformation Controlled and site-specific drug delivery Enhanced bio-absorption of encapsulated drug Improved tissue distribution and drug targeting via surface engineering Enhanced residence time of these carriers into mucosal linings such as gastrointestinal tract and ocular sites.
|
Low drug loading efficiency because of perfect crystalline structure Drug expulsion especially due to phase transition upon storage Initial burst release Large water content in nano-lipid dispersions
|
|
Nanostructured lipid carriers (NLCs)
|
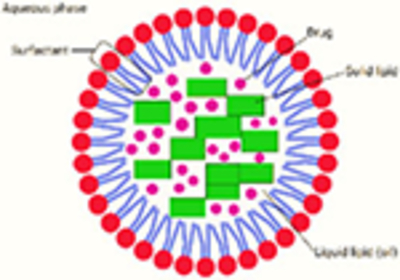
NLCs are modified versions of SLNs with better physical stability. Internal structure consists of both solid and liquid lipids. NLCs provide some imperfections in the core hence resulting into more stable preparation. One of the carriers of choice for topically applied drugs.
|
High entrapment of lipophilic drugs and hydrophilic drugs Increased dispersibility in an aqueous medium Extended release of the drug
|
Cytotoxic effects related to the nature of matrix and concentration Irritative and sensitizing action of some surfactants Encapsulation of protein and peptides still need to be exploited
|
|
|
Protein/polymer—nanoparticles
|
Albumin based
|
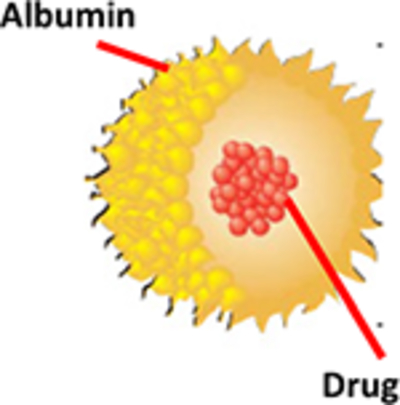
Albumin has high stability and high solubility in water and diluted salt solution. Excellent binding to lipophilic molecules Ovalbumin (OVA; derived from egg white), human serum albumin (HSA), bovine serum albumin (BSA), a binding to lipophilic molecules, and rat serum albumin (RSA) have been used for different biomedical applications (Karimi et al., 2016). Versatile carriers for different molecules and substances including drugs, genes, peptides, vaccines, and antibodies. Different geometrical shapes and structures include albumin NPs, albumin microspheres, albumin-coated liposomes, albumin microbubbles, and albumin nano capsules.
|
Faster distribution, higher Cmax, and greater AUC Selectively delivered to tumors by exploiting endogenous albumin pathways Low immunogenicity, no toxicity, greater drug uptake, and greater transcytosis across endothelial cells, good biocompatibility, and biodegradability HSA-based formulations alter plasma protein concentration and plasma colloid osmotic pressure.
|
Possible transmission of infectious agents (such as hepatitis, human immunodeficiency virus, etc.) and animal diseases. Heterogeneous nature of proteins reduce the possibility of reproducibility of final dosage form.
|
|
Polymeric nanoparticles
|
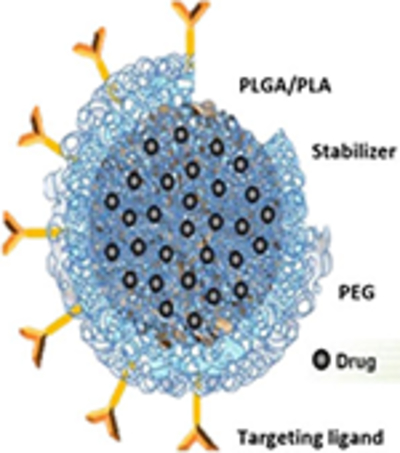
Three types based on drug encapsulation method: linear polymers (conjugation), polymeric micelles (synthesized amphiphilic block copolymers), and hydrogels (hydrophilic drug encapsulation). Rate of Release of therapeutic agents can be controlled Polymers can be natural (Chitosan, dextran, pectin, and alginate) and synthetic (PLGA, PCL, PLA are FDA-approved for biomedical application) Can be easily tailored and modified to desired characteristics like stimuli responsive.
|
Biodegradable, biocompatible and nontoxic Provides controlled release drug preserve the integrity of drug molecules and provide long-term stability
|
|
BIND-014 (PLGA-based) Phase 2 trial in patients with Metastatic Castration-Resistant Prostate Cancer |
Polymeric micelles
|
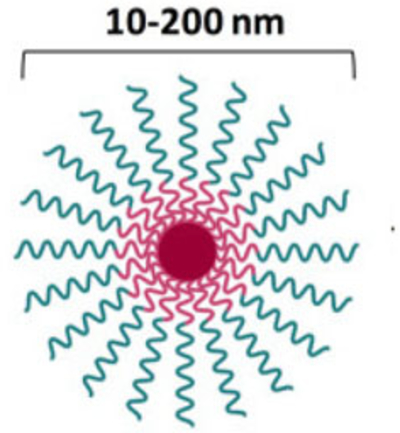
Size ranges from 10 to 100 nm. Formed from self-aggregation of amphiphilic polymers with the hydrophobic part of the polymer on the inside (core) and hydrophilic on the outside (shell); mPEG-PDLLA was used due to the ability to self-assemble at low critical micelle concentration Generally composed of block copolymers (deblock copolymers most apt) Poly(alpha-hydroxy esters), such as PDLLA, PGA, and PCL are the most widely employed polymers Core-shell structure of hydrophobic segments that serves as reservoir for the solubilization of hydrophobic drugs.
|
Hydrophilic shell provides protection in limiting opsonin adsorption and contributes toward a longer blood circulation time or better blood stability. Longer circulation time leads to improved accumulation at tissue sites with vascular abnormalities. Improve drug stability by inhibiting drug degradation.
|
Suitable only for poorly water-soluble drugs Low drug loading capacity Highly dependent on critical micelle concentration
|
Genexol PM® (Paclitaxel)
Nanoxel PM® (Docetaxel)
NK 105 (Paclitaxel)
SP1049C (doxorubicin) |
Dendrimers
|
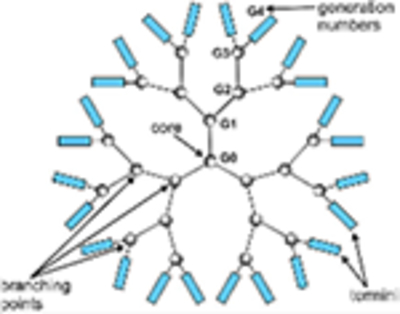
Basic structure of a dendrimer is composed of three different topological parts, namely a focal core, building blocks with several interior layers having repeating units, and multiple peripheral functional groups. Very small sizes (1–5 nm) possible by controlling the number of branches Low cytotoxicity and high permeability The most widely used dendrimers for biomedical applications are made of polyamidoamine (PAMAM) and polypropylene imine and Poly(amidoamine) organosilicon (PAMAMOS) dendrimers Used as a vehicle to deliver biologically active molecules such as vaccines, genes, and drugs, concomitantly. They can target tumor cells with drugs by incorporating tumor-affinity molecules (folic acid, anti-CD-14, prostate-specific membrane antigen, etc.). 3% of investigational NPs drugs since 2017 are being developed as dendrimers
|
Increasing dendrimer size and charge decreases and/or slows drainage from interstitial injection sites. PEGylation can enhance drainage from the interstitial and enhance parenteral bioavailability. Surface charge of dendrimers determines the nature of their interaction with membranes, cell surfaces, and plasma proteins. PAMAM dendrimers increase the Caco-2 permeability of drugs in the order cationic > anionic > uncharged or PEGylated Improved efficacy and safety, improved solubility, improved pharmacokinetics, benefit in combination with marketed anti-cancer therapies
|
|
VivaGel™ (SPL7013) AZD0466 (AZD4320-dendrimer conjugate): Phase I trial NCT04214093 DEP® Docetaxel (detergent-free formulation of docetaxel): Phase 2 clinical trial starting DEP® Irinotecan (Irinotecan)
|








